(1) Knoop experiment
The oxidative breakdown of fatty acids can be carried out in various tissue cells in animals and is one of the important sources of energy supply for cells.
Knoop (1904) uses phenyl, which is not easily degradable in vivo, as a marker to attach to the methyl ends of fatty acids, and then feeds dogs or rabbits. It was found that, if fed benzene ring labeled even carbon atom fatty acids, the metabolite in animal urine is phenylacetic acid, such as feeding benzene ring labeled odd carbon atom fatty acids, the metabolite found in the urine is benzoic acid.
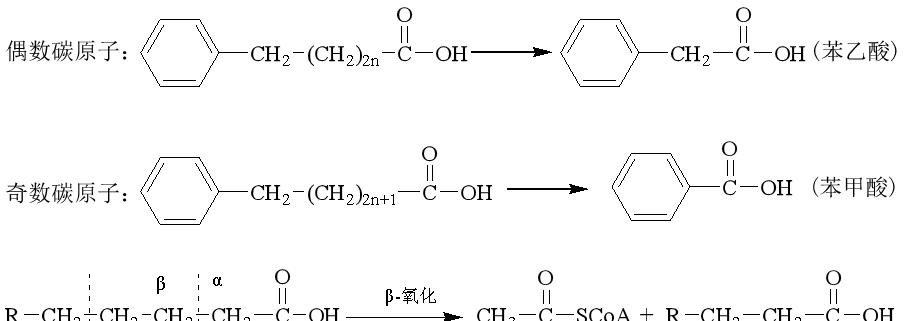
Based on this, he proposed that the oxidative decomposition of fatty acids in the body begins with β-carbon atoms at the carboxyl end, and the carbon chains are broken successively, each time producing a two-carbon unit, that is, acetyl CoA. This is the "β-oxidation theory".
(2) β-oxidation of fatty acids (mitochondria)
(1) Activation of fatty acids Fatty acids are activated in the cell fluid into lipidyl CoA.
(2) Transfer of lipidyl CoA from the cytosol to the mitochondria Its transfer is achieved by means of a lipoayl carrier- carnitine (carnitine). The molecular formula of carnitine is:
L-(CH3)3N+- CH2CH(OH)CH2COO-L-β-hydroxy-γ-trimethylaminobutyric acid;
The transfer of lipid CoA into mitochondria is the main rate-limiting step in fatty acid β-oxidation, and carnitine lipacyltransferase I is its rate-limiting enzyme. When the fat mobilization effect is strengthened, the body needs fatty acids to provide energy, at this time the activity of carnitine alicyl transferase I increases, the oxidation of fatty acids is enhanced, and when fat synthesis, malonate monoacyl CoA inhibits the activity of this enzyme.
Since each β-oxidation is carried out, acetyl CoA, FADH2 and NADH+H+ are generated. Palmitic acid is a saturated fatty acid of hexadecicarbon that undergoes a total of 7 β-oxidation processes, and its total reaction is as follows:
Produces 106 ATP.