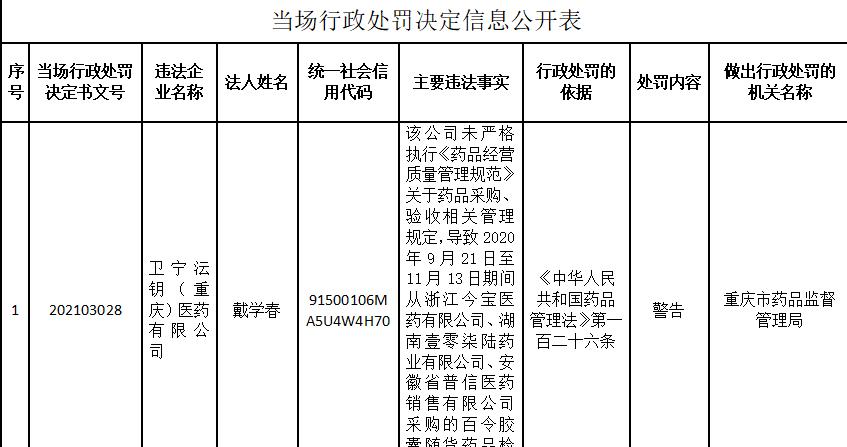
China Economic Network Beijing, December 15, 2020 Chongqing Municipal Drug Administration website recently published on the spot administrative punishment decision information disclosure form shows that Weining Key (Chongqing) Pharmaceutical Co., Ltd. did not strictly implement the "Drug Business Quality Management Practice" on drug procurement, acceptance of the relevant management regulations, resulting in from Zhejiang Jinbao Pharmaceutical Co., Ltd., Hunan Yizhiqilu Pharmaceutical Co., Ltd. from September 21, 2020 to November 13, 2020, The inspection report of the Bailing capsules purchased by Anhui Puxin Pharmaceutical Sales Co., Ltd. is suspected of being forged. The Chongqing Municipal Drug Administration warned him in accordance with Article 126 of the Drug Administration Law of the People's Republic of China.
After an inquiry by a reporter from China Economic Network, it was found that Weining Key (Chongqing) Pharmaceutical Co., Ltd. is a wholly-owned subsidiary of Shanghai Dilin Biotechnology Development Co., Ltd., Shanghai Dilin Biotechnology Development Co., Ltd. is a wholly-owned subsidiary of Weining Key Technology (Shanghai) Co., Ltd., and the largest shareholder of Weining Key Technology (Shanghai) Co., Ltd. is Weining Health ("Weining Health", 300253.SZ), with a shareholding ratio of 45.70%.
Weining Key Technology (Shanghai) Co., Ltd., formerly known as "Shanghai Key Circle Cloud Health Technology Development Co., Ltd.", has been renamed in July 2021, Weining Health Annual Report shows that key circle company is not under the same control of the merger of enterprises, as the company's cloud drug platform, actively promote the construction, layout and operation of the prescription circulation platform, and continue to optimize the development and pilot of electronic prescription safety management and circulation platform. At the same time, it has achieved system docking, variety matching and inventory synchronization with mainstream distributors and retailers of relevant pharmaceutical and health products, and is committed to creating an off-campus drug and health product supply chain of "open, China joint network communication, win-win and neutral". After the key world circle is included in the scope of the company's consolidated statements, the company will strengthen the strategic support for the key world circle, increase the investment, grafting and coordinated development of various resources in the system, and further promote the landing and rapid expansion of weining cloud medicine strategy.
Article 126 of the Drug Administration Law of the People's Republic of China stipulates: Except as otherwise provided in this Law, if the holder of the drug marketing authorization, the drug production enterprise, the drug trading enterprise, the drug non-clinical safety evaluation research institution, the drug clinical trial institution, etc. fail to comply with the drug production quality management standards, drug business quality management standards, drug non-clinical research quality management standards, drug clinical trial quality management standards, etc., they shall be ordered to make corrections within a time limit and give a warning; Impose a fine of between 100,000 and 500,000 yuan; if the circumstances are serious, impose a fine of between 500,000 and 2 million yuan, order the suspension of production and business for rectification until the drug approval documents, drug production licenses, drug business licenses, etc. are revoked, and drug non-clinical safety evaluation research institutions, drug clinical trial institutions, etc. must not carry out drug non-clinical safety evaluation research and drug clinical trials within five years, and the legal representative, the main responsible person, the directly responsible supervisor and other responsible personnel shall be punished, Confiscate the income obtained from the unit during the illegal act, and impose a fine of not less than 10% to 50% of the income obtained, and prohibit him from engaging in drug production and operation and other activities for 10 years until life.
The following is the original text: