Approximately 30%-50% of Asian non-small cell lung cancer (NSCLC) patients carry egfr mutations, and current drugs for such mutations are:
Generation: gefitinib (Iressa), Erlotinib (Trocai), Exetinib (Kemena);
Second generation: afatinib (geteri), daktinib (Dozerun);
Three generations: osimitinib (teresa), ametinib (amele).
So what to do after three generations of drug resistance? Take a look at the image below.
Tip: Please view your phone in landscape.
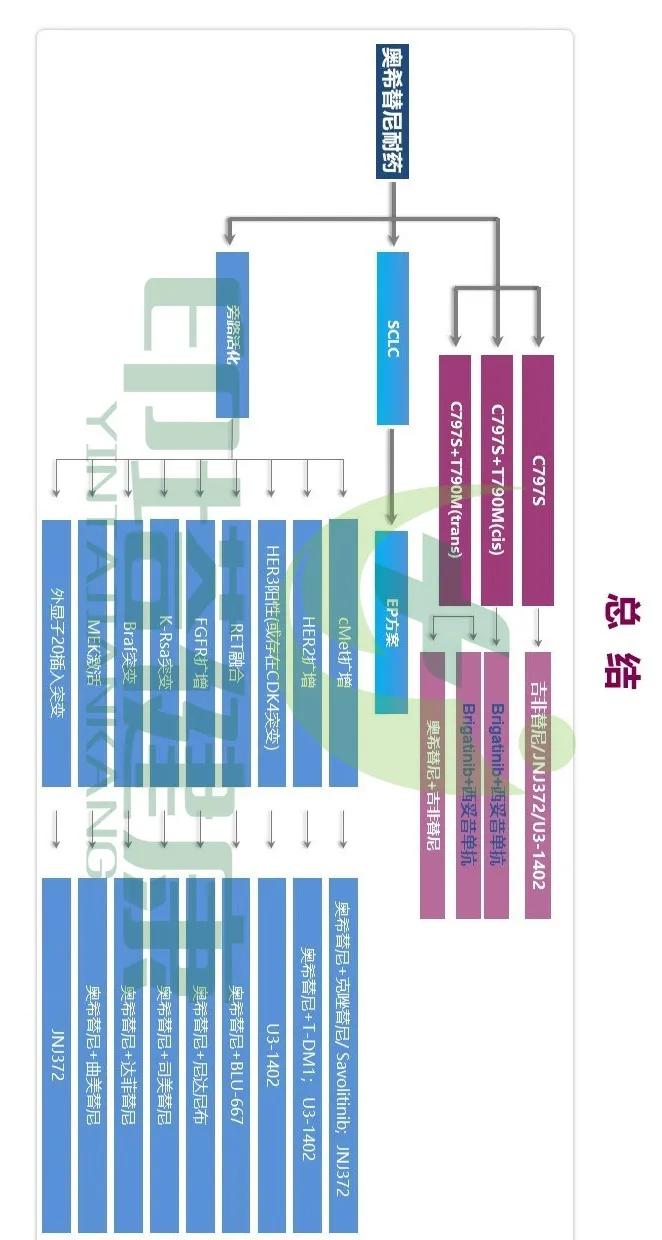
After oscitinib resistance, the t790m/c797s mutation is the most common mutation. The analysis of resistance in the aura3 study on second-line treatment with osimertinib is as follows:
Acquired egfr mutations: 21%;
met amplification: 19%;
Cell cycle gene changes: 12%;
Her2 amplification: 5%;
PIK3CA amplification/mutation: 5%;
Oncogene fusion: 4%;
braf v600e: 3%。
T790m/c797s cis-mutation (cis-) means that two genes are on the same DNA strand, and t79om/c7975 trans-mutation (trans-) is that two genes are located on different DNA strands.
Bugatinib is a novel dual inhibitor of alk and egfr that inhibits L1196m mutation of ALK and T790m mutation of EGFR. Cetuximab (epitimal) is an iigg1 monoclonal antibody that targets the epidermal growth factor receptor (egfr).
Note: Bangladesh beacon (Beacon) bugatinib 90mg
Product Name:alunbrig
Common name: bugatinib (brigatinib)
Targets: alk, egfr
Manufacturer: Arriad (ariad is a wholly-owned subsidiary of Takeda Pharmaceutical)
First approval in the United States: April 2017
China's first approval: not approved
Specifications: 30mg*21, 30mg*180, 90mg*60
Approved indications: alk-positive metastatic NSCLC of kzotinib intolerance or disease progression after medication, ALK-positive NSCLC of first-line treatment
Recommended dose: Initial dose: 90 mg orally every day for the first 7 days. If the initial dose is tolerated in the first 7 days, it is taken orally once a day, the dose is increased to 180 mg. Serve on an empty stomach or with meals.
In April 2020, the Journal of Thoracic oncology published a retrospective cohort study of 15 patients with non-small cell lung cancer (nsclc) who developed cis-mutations of egfr (19del or l858r)/t790m/c797s who were on the second line or later on osiminib ≥ for 2 months and developed egfr (19del or l858r)/t790m/c797s.
Five of these patients received targeted combination therapy: one cycle every 21 days, with intravenous cetuximab 500 mg/m2 administered intravenously on days 1 and 8, while oral bugatinib was given once a day. The dose of bugatinib for the first 7 days is 90 mg, if the patient tolerates it, the dose is increased from day 8 to 180 mg.
10 patients received chemotherapy: every 28 days for a cycle, intravenous cisplatin 75 mg/m2 combined with pemetrexed 500 mg/m2 or paclitaxel 175 mg/m2, of which 6 patients received bevacizumab 15 mg/kg at the same time.
Photo note: Bangladesh beacon (Bikang) bugatinib 180mg
Clinical data
Among the 5 patients (all with cis mutations egfr19del/t790m/c797s) who received bugatinib plus cetuximab, 3 patients had partial remission (pr), 2 were disease stable (sd), the objective response rate (orr) was 60%, and the disease control rate (dcr) was 100%. By the time of data cut-off, 3 patients were still receiving treatment, and 2 patients had disease progression (PD), with pfs at 15 and 13 months, respectively.
Of the 10 patients who received chemotherapy, 1 was PR, 5 were SD, 4 were PD, orr was only 10%, and DCR was 60%.
The median PFS in the bugatinib + cetuximab vs chemotherapy groups was 14 months vs 3 months.
No grade III or IV adverse reactions (ae) were observed in all patients, and 4 patients in the bugatinib + cetuxib group and 6 patients in the chemotherapy group developed grade I or II adverse reactions. One patient reduced the dose of bugatinib to 120 mg due to grade I fatigue and vomiting. In patients undergoing chemotherapy, leukopenia (n=5) is the most common adverse reaction.
Bugatinib plus cetuximab combination therapy is an effective treatment strategy that can improve the survival outcome of patients with triple resistance mutations of cis-egfr/t790m/c797s that appear after osimtinib resistance!
【Important】This public number [Global Good Medicine Information] All article information is for reference only, and the specific treatment is in accordance with the doctor's advice!