Text / Inquiry Bio Please move to the comment area for consultation
<h1 class="pgc-h-arrow-right" data-track="2" > nitrogen cycle in water</h1>
The main links constituting the nitrogen cycle are: synthesis, ammonization, nitrification, denitrification and nitrogen fixation in living organisms. The nitrogen in the natural water body comes from the accumulation and decay of aquatic animal and plant corpses and excreta, nitrogen-containing organic compounds are decomposed into small molecules such as ammonia nitrogen and hydrogen sulfide by saprophytic bacteria, and then converted into nitrite and nitrate by various autotrophic microorganisms mainly for the role of nitrifying bacteria, these three nitrogens are absorbed by algae and aquatic plants on the one hand, and on the other hand, nitrate is converted into nitrogen escape water by denitrifying bacteria under hypoxic conditions. Nitrogen in the atmosphere is used by nitrogen-fixing bacteria to return to the water body.
Due to the different growth and reproduction rates of various microorganisms, in the entire nitrogen conversion process, the conversion from nitrogen-containing organic matter to ammonia nitrogen is performed by a variety of heterotrophic microorganisms, and the growth and reproduction of such microorganisms is faster, so the process time is shorter; from ammonia nitrogen to nitrite conversion is performed by nitrosinizing bacteria, the growth and reproduction rate of nitrosinous bacteria is 18 minutes a generation, so its transformation time is also shorter; from nitrite to nitrate is served by nitrate bacteria, and the growth rate of nitrate bacteria is relatively slow, Its reproductive rate is 18 hours a generation.
Therefore, the time to convert from nitrite to nitrate is much longer, and the effective decomposition of nitrosinous nitrogen takes 12 days or more.
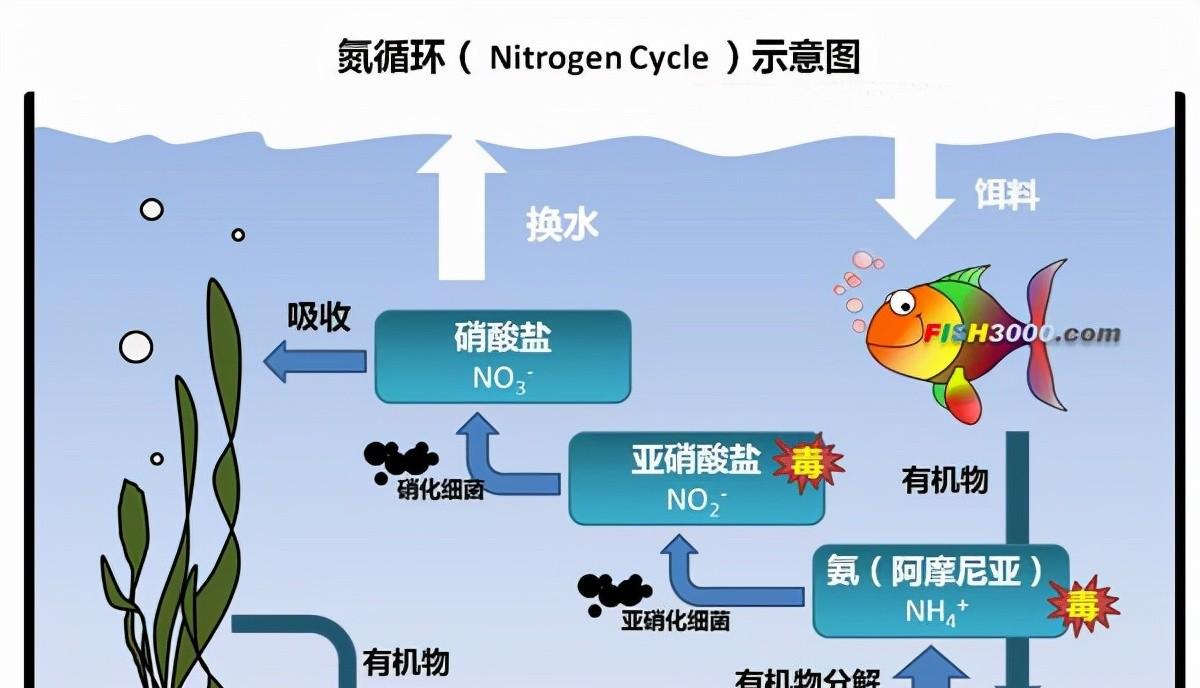
<h1 class="pgc-h-arrow-right" data-track="7" > the accumulation and toxicity of ammonia nitrogen and nitrous nitrogen in aquaculture waters</h1>
Under normal circumstances, the nitrogen cycle of the water body is in a stable state, and the ammonia nitrogen and nitrous nitrogen in the water body maintain normal levels. In the water body of high-density breeding and freshwater integrated aquaculture, due to the accumulation of a large amount of residual bait left by bait and a large amount of excrement of aquatic animals in the water body, and the regular use of disinfectants, while killing harmful microorganisms, the types and quantities of beneficial microorganisms will be correspondingly reduced, the water ecological imbalance is manifested as deterioration of water quality, reduced transparency of water bodies, hypoxia of water bodies, obstruction of a large amount of accumulated nitrogen nitrification process, and high ammonia nitrogen and nitrite content in aquaculture water bodies, especially when the temperature and pH are low, Nitrification weakens, resulting in more pronounced accumulation of nitrite.
The total ammonia in water bodies includes molecular ammonia (NH) and ionic ammonia (NH), of which molecular ammonia is the one that has a significant toxic effect on fish. With the difference of pH, the two can be converted into each other in water, and the ratio of molecular ammonia to ionic ammonia in water is closely related to water temperature and pH. Overall, temperature and pH values rise, the proportion of free ammonia in total ammonia increases, and the more free ammonia is found, the more toxic it becomes. The maximum permissible concentration of ionic ammonia in cultured waters is not more than 5 mg of nitrogen per liter (5 mgN/L), while the maximum allowable concentration of molecular ammonia is only 0.1 mg of nitrogen per liter (0. 1 mgN/L)。 Regarding the toxic effect of ammonia, it is generally believed that the molecular ammonia that penetrates into the organism oxidizes the Fe2+ of the hemoglobin molecule in the blood into Fe3+, reducing the oxygen carrying capacity of the blood and reducing the respiratory function. It can be seen that the lower the dissolved oxygen of the water, the more intense the ammonia toxicity. Ammonia mainly invades the mucous membranes, especially the gills epidermis and intestinal mucosa, followed by the nervous system, causing damage to the liver and kidney system of aquatic animals such as fish, causing hyperemia of the body surface and internal organs, muscle hyperplasia and tumors, and serious liver coma and death. Even at low concentrations of ammonia, long-term exposure can damage gill tissue, with gill flakes bending, sticking or fusing.
Nitrite is an intermediate product that cannot be completely carried out by nitrification reactions, and when the total ammonia concentration of the water body reaches a peak for 3 to 4 days, the nitrite concentration also increases and peaks accordingly. Compared with ammonia poisoning, nitrite is less toxic to fish and shrimp, but due to the rapid conversion rate of ammonia nitrogen, the problem of nitrite is the most prominent. The mechanism of action of nitrite is similar to that of ammonia nitrogen poisoning, mainly through the respiration of fish and shrimp from the gill wire into the blood, which can oxidize normal hemoglobin into high-value hemoglobin and reduce the oxygen-carrying function of proteins that transport oxygen. Tissue hypoxia occurs, fish and shrimp feed is reduced, gill tissue lesions occur, breathing difficulties, restlessness or slow response, resulting in hypoxia or even suffocation death of fish and shrimp. Nitrites can also react with secondary amines to form carcinogenic nitrites, which are conducive to nitrite formation at low pH. Many ponds have anorexia of fish and shrimp, and high nitrite is one of the main reasons.
<h1 class="pgc-h-arrow-right" data-track="12" > biological regulation of ammonia nitrogen in cultured waters</h1>
At present, the methods of reducing ammonia nitrogen in aquaculture water include chemical redox method, physical adsorption method or open pump oxygenation method, biological fertilizer water and bacterial decomposition method. The long-term use of the first two methods will change the nature of the pond sediment, and can not fundamentally solve the problem, and the biodegradable water ammonia nitrogen, nitrosinite nitrogen is to rely on the regulation of biological factors in the water body (algae and microorganisms) to effectively transform the organic pollutants in the water body, to achieve self-purification, is conducive to the establishment of a reasonable aquatic ecological cycle, is an effective method of healthy aquaculture water quality regulation.
2.1 Mechanism of purification of microalgae on water bodies and study on the removal of ammonia helium in aquaculture water bodies
Microalgae, also known as single-celled algae, are tiny algal populations that can only be identified under the microscope, accounting for about 70% of the more than 30,000 known algae species worldwide. Microalgae are self-nourishing organisms with light energy as an electron donor, using light energy as energy source, and using nutrients such as nitrogen and phosphorus to synthesize complex organic matter. Nitrates, nitrites and ammonium salts absorbed by algae cells can be used for the synthesis of nitrogen-containing substances such as amino acids and proteins, chlorophyll, etc., and microalgae provide bait for a variety of fish, so the growth of microalgae can reduce the nitrogen and phosphorus content in water. The microalgae with the best effect on nitrogen and phosphorus absorption are spirulina, chlorella, gravis, fibrillary algae, grating algae, etc., especially chlorella has the strongest nitrogen reduction ability.
Inoculation of beneficial algae in the cultured water body can not only play the role of nitrogen removal and oxygenation, but also play the role of bait fertilizer water, and when it forms a dominant group, it can also inhibit the growth of harmful algae (microcystis). The best water color suitable for fish farming in aquaculture is oil green (the main species of phytoplankton are cryptoalgae, diatoms, golden algae and chlorella green, etc.) and light brown (the main species of phytoplankton are diatoms, golden algae, yellow-green algae, etc.), and the algae contained in these two types of water are easily digested and absorbed by fish, and are very good natural food for fish and other farmed species. The photosynthesis of algae can also produce a large amount of oxygen, and it is reported that 80% of the dissolved oxygen in the water comes from the photosynthesis of algae. Sufficient oxygen can promote the conversion of nitrite to nitrate, at the same time, it can reduce the foul odor formed by the lack of oxygen in the water body, improve the ecological environment of the water body, inhibit and reduce the toxic effect of ammonia nitrogen, nitrite and hydrogen sulfide on fish, improve fish appetite and feed utilization, and promote fish growth and development.
2.2 Research and application status of microecological agents in freshwater aquaculture
Microecological preparation nitrifying bacteria is an active bacteria preparation containing a large number of beneficial bacteria made after the microbial bacteria selected from the natural environment are cultured and multiplied, and it is a new type of bait additive developed in recent years. The aquaculture water environment itself is a dynamic equilibrium system composed of a variety of microorganisms, and beneficial bacteria and harmful bacteria coexist. Numerous studies have shown that when beneficial microorganisms are added to water bodies, the dominant population can inhibit the growth of harmful bacteria through large numbers of breeding, and at the same time, through the metabolism of beneficial microorganisms, excess nutrients and other harmful substances in water can be reduced, which has obvious regulatory effects on removing ammonia nitrogen, organic matter, reducing BOD, COD and increasing dissolved oxygen in water bodies, and also regulates the pH of water bodies and promotes the release of nitrogen and phosphorus in the sediment to promote the growth of plankton.
Originated from the world's leading Danish nitrifying bacteria biotechnology and strains, it can quickly eliminate ammonia nitrogen and nitrite in water, purify water quality, and promote the rapid establishment of nitrifying bacteria in cultured water. In general, ammonia nitrogen can be reduced below the safe value after 12 hours of use, and nitrite can be reduced below the safe value after 48 hours.
This product has passed the evaluation of microbial agent and ecological safety, and is not affected by environmental factors such as organic matter, water quality and pH of aquaculture water. There are no adverse effects on water beneficial bacteria, algae, plankton, and farmed aquatic animals.
Recommended dosage: 300-500ml per mu once a week in the early stage of breeding. When ammonia nitrogen and nitrite exceed the standard, it is 500-1000ml per acre, and it is used twice a day. When high-density aquaculture ammonia nitrogen and nitrite are seriously exceeded, 10ml per cubic water body, once a day, and used 2-3 times.
Do not use with antibiotics, disinfectants, pesticides and strong acids and alkalis.