At present, there are 5 CAR-T products approved by the FDA worldwide to treat many different types of hematologic malignancies. CAR-T has been making great progress in the field of hematological tumors, while in the field of solid tumors, which account for more than 90%, CAR-T therapy has made slow progress and is difficult.
In view of the "dilemma" of CAR-T solid tumors, this paper summarizes the four key challenges currently facing us and the latest recent research progress.
01
CAR-T's solid tumor "dilemma"
CAR-T's entry into solid tumors mainly faces the following major challenges:
1.1 Lack of effective targets
Unlike hematological tumors, which are mostly single and specific, tumor-specific antigens (TSAs) are rare in solid tumors, and most of the highly expressed antigens found in tumors are tumor-associated antigens (TAA) that are also expressed in normal tissues. This creates a high risk of off-target, and safety is a key concern.
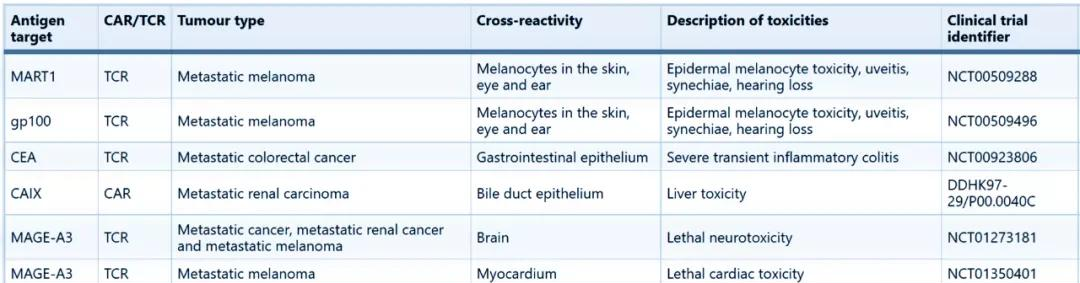
Source: Nature Reviews Drug Discovery
The chart above shows Nature Reviews Drug Discovery summarizing the serious adverse reactions due to "on target, off-tumour" in recent clinical trials of T cell therapy. At the same time, solid tumors are highly heterogeneous, and even if there is a safe target, whether the efficacy can be guaranteed is also a big challenge.
1.2 Transport and infiltration of CAR-T
Solid tumors have mechanisms that interfere with T cell transport for immune escape. On the one hand, unlike blood tumor cells that are scattered, solid tumors tend to form solid clumps, which, together with abundant tumor-associated fibroblasts (CFFs) and blood vessels, form a natural physical barrier.
On the other hand, some solid tumors inhibit the secretion of certain chemokines. The interaction of chemokines with their receptors facilitates the migration of T cells to the tumor microenvironment. At the same time, the surface of CAR-T cells also lacks related receptors that match the chemokines secreted by solid tumors, resulting in poor nesting ability of CAR-T to tumor sites.
1.3 Immunosuppression of the tumor microenvironment
The study found that the low pH, low oxygen, high penetration in the tumor microenvironment (TME), and the existence of immunosuppressive mechanisms are extremely detrimental to the survival and immune efficacy of T cells.
External environment that limits CAR-T's antitumor effects (Source: Nature Reviews Drug Discovery)
Immunosuppressive cells such as regulatory T cells (Treg), bone marrow-derived heterogeneous cells (MDSCs), and M2 macrophages are present in TME. These immunosuppressive cells release cytokines such as transforming growth factor β (TGFβ) and interleukin-10 (IL-10) in solid tumors, reducing the anti-tumor effect of CAR-T after reinfusion.
1.4 Endogenous T cells inhibit signaling
There is an endogenous regulatory mechanism in the immune activity of T cells, and when T cells are overactive, molecules such as PD-1 and CTLA4 play a role in maintaining immune balance. After CAR-T cells are activated by antigens, PD-1 and CTLA4 bind to associated ligands, inhibiting the proliferation of T cells and the secretion of related cytokines. Therefore, endogenous T cell inhibition signaling also reduces the antitumor activity of CAR-T.
02
CAR-T new strategies against solid tumors
On the road to combating solid tumors, CAR-T faces multiple dilemmas, and the following summarizes some of the new breakthroughs of CAR-T in solid tumors for the above mechanisms.
NEW CAR-T STRATEGY FOR DEALING WITH SOLID TUMORS (Source: Cancers)
2.1 Improve the recognition of CAR-T antigens
Common TAA targets in solid tumors include CEA, HER2, GPC3, EpCAM, etc., and there are fewer TSA targets, which seriously limits the application of CAR-T in solid tumors. In addition to continuing to search for and develop CAR-T therapies for TSA targets, CAR-T cells can also be engineered to improve the recognition of tumor antigens.
April 28, . Professor WendellLim's team implanted the synNotch system in CAR-T cells, and under the regulation of synNotch, the CAR that recognizes the associated TAA is only expressed on T cells migrating to the tumor, and does not attack the cells in normal tissues, greatly improving the specificity of CAR-T recognition antigens. (Science Translational Medicine, 2021, 13, 7378)
Multi-antigen-and-kill loops in T cells provide a universal strategy for overcoming antigenic heterogeneity while still maintaining high tumor specificity (Source: Science Translational Medicine)
Recently, the team of Carl H. June, the godfather of CAR-T therapy, and Andy J. Minn, a professor of radiation oncology at Perelman School of Medicine, have also discovered a new way to improve CAR-T's recognition of tumor cells," equipping CAR-T with a variety of new "weapons" that make CAR-T no longer "fight alone." Multi-armored CAR-T improves infiltration and recognition of tumor cells and, in the case of antigen loss, initiates endogenous CAR-T to clear the tumor. (Cell,2021, 184, 19, 4981-4995)
"Multi-armored" CAR-T can significantly improve the anti-cancer effect (Source: Cell)
In addition, in addition to modifying the CAR-T cells themselves, scientists also used external conditions to improve the specific recognition of CAR-T tumor cells. On August 12, researchers at the University of California, San Diego, published a research advance in Nature Biomedical Engineering, which can destroy tumor tissue while protecting normal tissue, greatly improving the safety of CAR-T therapy. (Nature Biomedical Engineering,doi.org/10.1038/s41551-021-00779-w)
2.2 Multi-target combination
At present, bispecific CAR-T for two targets has been applied in the field of hematological tumors, which can improve the recognition ability of CAR-T antigens, increase efficacy and safety, and reduce the risk of tumor escape. CAR-T therapies that target two or more different TAAs are also important for the treatment of solid tumors.
On September 23, Nature Cancer published an advance in two-target CAR-T research on a model of neuroblastoma (NB). The study targeted two NB-associated antigens, GD2 and B7-H3, and provided CD28 and 4-1BB co-stimulation, achieving rapid and sustained antitumor effects in mice and preventing tumor immune escape due to low antigen density. (Nature Cancer. 2021, 2, 904–918)
This experimental strategy prevents tumor escape due to antigen loss (Source: Nature Cancer)
2.3 Improve infiltration and transport of T cells
Intravenous reintroduction is usually used for the treatment of hematological tumors, and in solid tumors, intratumoral administration can be used to improve the infiltration and transport of CAR-T. Intratumoral administration has been well achieved in the study of pleural malignant mesothelioma, head and neck cancer and malignant glioma.
Chemokines are important molecules that affect T cell infiltration, and the study of chemokines in recent years has brought new hope to CAR-T to overcome solid tumors. On September 22, the R&D team of Guangzhou Baiji Gene for the first time modified the chemokine receptor CXCR5 to the surface of CAR-T cells targeting EGFR for the treatment of non-small cell lung cancer (NSCLC). The results of in vivo CAR-T tracing experiments showed that CXCR5-modified CAR-T cells could migrate and penetrate to the tumor lesion in a targeted manner, and significantly cleared the tumor, while greatly reducing the potential extranoplastic toxicity. (Molecular Therapy-Oncolytics , 2021, 22,507-517)
In vivo antitumor activity of CAR-T cells (Source: Molecular Therapy-Oncolytics)
In addition, there are also studies that target fibroblast activated proteins (FAPs) expressed in tumor stromal cells, which can block the formation of stromal and enhance CAR-T infiltration of solid tumors.
2.4 Release immunosuppression
The main reasons for the immunosuppressive effect of TME are immunosuppressive cytokines, the presence of immunosuppressive cells, and the absence of immunoactive factors. The immunosuppressive effect in TME is one of the key factors influencing the effectiveness of CAR-T in solid tumors.
Some studies have modulated local microenvironments by modifying CAR-T cells to overexpress inflammation-promoting cytokines such as IL-12, IL-15, and IL-18, which are called "armored" CAR-T cells. Studies have confirmed that in ovarian cancer, IL-12 can improve the proliferation and viability of T cells, resist apoptosis and PD-1-induced functional inhibition. In addition, studies of CAR T cells secreting IL-18 have shown that these cells have improved ability to proliferate and infiltrate, and can recruit endogenous immune cells to regulate TME.
Reduces immunosuppression in TME (Source: American Journal of Cancer Research)
At the same time, since TGFβ is an important immunosuppressive pathway, blocking TGFβ signaling can also improve the anti-tumor effect of CAR-T.
In addition to the immunosuppressive effect in TME, T cells also have endogenous immunosuppressive mechanisms. At present, gene silencing, PD-1 switching receptors, and combination with PD-1 inhibitors are mainly used to avoid endogenous inhibitory signals.
Release of endogenous T cell inhibitory signals (Source: American Journal of Cancer Research)
03
Combined is the future direction
Although the response rate of CAR-T after treatment of hematologic malignancies is high, it is still a challenge to achieve sustained remission, and patients often have the phenomenon of disease recurrence. The efficacy of CAR-T against tumors is affected by various factors such as the activation and expansion of T cells in vivo, killing effect and persistence, and is also closely related to the tumor type and individual patient differences. In the face of highly complex solid tumors, it is difficult to balance these factors with a single CAR-T therapy.
In addition to enhancing the killing effect of CAR-T itself on tumor cells, efforts should also be made to explore strategies to deal with difficult problems such as CAR-T amplification and persistence. CAR-T combined with traditional radiotherapy and chemotherapy, immune checkpoint inhibitors, vaccines and oncolytic viruses show broad application prospects, and is expected to achieve the effect of treating solid tumors by directly enhancing T cell function, recruiting endogenous immune cells and remodeling TME.
Resources:
[1] Andrew J. Hou et al. Navigating CAR-T cells through thesolid-tumour microenvironment. Nature Reviews Drug Discovery. (2021)
[2] Yuna Jo. Innovative CAR-T Cell Therapy for Solid Tumor; CurrentDuel between CAR-T Spear and Tumor Shield. Cancers. (2021)
[3] Zheng Biao's review | Science Breakthrough: New CAR-T Effectively Kills Solid Tumors without Harming Normal Cells (Source: Rubik's Cube Pro)
[4] Koichi Hirabayashi et al. Dual-targeting CAR-T cells with optimalco-stimulation and metabolic fitness enhance antitumor activity and preventescape in solid tumors. Nature Cancer. (2021)
[5] Guangchao Li et al. CXCR5 guides migration and tumor eradication ofanti-EGFR chimeric antigen receptor T cells. Molecular Therapy-Oncolytics. (2021)
[6] Meijuan Huang et al. Innovative strategies to advance CAR T celltherapy for solid tumors. American Journal of Cancer Research.(2021)
[7] Joseph H. Choe et al. SynNotch-CAR T cells overcome challenges ofspecificity, heterogeneity, and persistence in treating glioblastoma. (2021)
[8] Lexus R. Johnson et al. The immunostimulatory RNA RN7SL1 enablesCAR-T cells to enhance autonomous and endogenous immune function. Cell (2021).
[9] Yiqian Wu et al. Control of the activity of CAR-T cells withintumours via focused ultrasound. Nature Biomedical Engineering. (2021)
[Yao Zheng et al., Research progress of CAR-T cell immunotherapy for solid tumors. Journal of China Pharmaceutical University (2021)
[11] Tian Yonggui et al., "Research progress of next-generation car telecommunications in the treatment of solid tumors. Chinese Journal of Immunology (2020)