Sulfur dioxide is the most common, simplest, and irritating sulfur oxide, chemical formula SO2, colorless and easily soluble in water gas, one of the main pollutants in the atmosphere. The gas erupts when a volcano erupts, and sulfur dioxide is also produced in many industrial processes. Since coal and oil usually contain sulfur, sulfur dioxide is formed when burned. When sulfur dioxide is dissolved in water, sulfurous acid is formed. If sulfurous acid is further oxidized under the condition of PM2.5, sulfuric acid (the main component of acid rain) will be quickly and efficiently generated. This is one of the reasons for concern about the environmental effects of using these fuels as an energy source. At present, many places with sulfur dioxide must install an online sulfur dioxide detector for 24-hour real-time online detection to ensure the safety of workers every minute and second, when the gas concentration exceeds the standard, it will automatically issue an alarm and open the exhaust fan and spray device at the same time, timely remind people and eliminate safety hazards.
On October 27, 2017, the List of Carcinogens released by the International Agency for Research on Cancer of the World Health Organization preliminarily collated and referred to sulfur dioxide in the list of class 3 carcinogens
Sulfur dioxide is bleached. Industrially, sulfur dioxide is commonly used to bleach pulp, wool, silk, straw hats, etc. The bleaching effect of sulfur dioxide is due to its (sulfurous acid) ability to form unstable colorless substances with certain colored substances. This colorless substance is easy to decompose and the colored substance returns to its original color, so the straw hat braid that has been bleached with sulfur dioxide turns yellow over time. The bleaching effect of sulfur dioxide and some sulfur-containing compounds is also illegally used by some unscrupulous manufacturers to process food to whiten food. Eating such foods has serious damage to the liver and kidneys of the human body, and has a carcinogenic effect
In addition, sulfur dioxide can also inhibit the growth of mold and bacteria, and can be used as a preservative for food and dried fruits. However, it must be used in strict accordance with the relevant national scope and standards.
Let's take a good look at it and understand it
Physical properties: Sulfur dioxide is a colorless transparent gas with a pungent odor. Soluble in water, ethanol and ether
Chemical properties: At room temperature, humid sulfur dioxide reacts with hydrogen sulfide to precipitate sulfur. At high temperatures and in the presence of catalysts, hydrogen can be reduced to hydrogen sulfide and carbon monoxide to sulfur. Strong oxidants can oxidize sulfur dioxide to sulfur trioxide, and oxygen can oxidize sulfur dioxide to sulfur trioxide only in the presence of a catalyst. It has spontaneous combustion and no combustion. Liquid sulfur dioxide can dissolve organic compounds such as amines, ethers, alcohols, phenols, organic acids, aromatic hydrocarbons and other organic compounds, and most saturated hydrocarbons cannot be dissolved. It has a certain degree of water solubility, and reacts with water and water vapor to generate toxic and corrosive vapors.
Application field industry: mainly used for the production of sulfur trioxide, sulfuric acid, sulfite, thiosulfate, also used as fumigants, preservatives, disinfectants, reducing agents, etc., can also be used as organic solvents and refrigerants, and used to refine a variety of lubricating oils, or the production of sulfur and as insecticides, fungicides.
Contact control
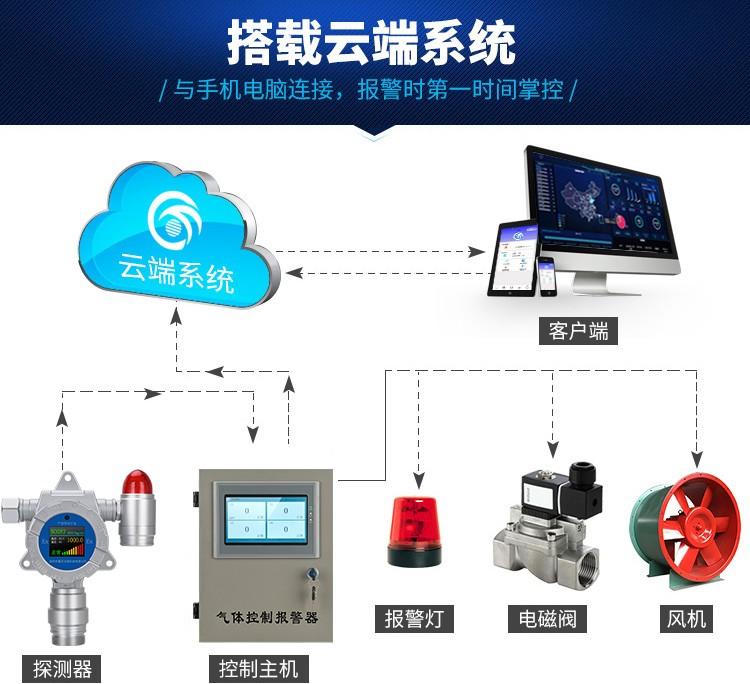
Monitoring method: On-line 24-hour monitoring using sulfur dioxide gas detector, portable and stationary
Engineering control: strictly sealed, providing sufficient local exhaust and comprehensive ventilation. Safety shower and eye washing facilities are provided.
Respiratory protection: When the concentration in the air exceeds the standard, wear a self-priming filter gas mask (full-face mask). In case of emergency rescue or evacuation, it is recommended to wear a positive pressure self-contained respirator.
Eye protection: Respiratory protection has been provided.
Body protection: Wear polyethylene poison suits.
Hand protection: Wear rubber gloves.
Other protection: Smoking, eating and drinking are prohibited on the work site. When you're done, change clothes in the shower. Practice good hygiene
Precautions
In the atmosphere, sulfur dioxide oxidizes into sulfuric acid mist or sulfate aerosols, which is an important precursor to environmental acidification. Atmospheric sulfur dioxide concentrations above 0.5 ppm have potential effects on the human body; most people begin to feel irritated at 1 to 3 ppm; at 400 to 500 ppm, people will have ulcers and pulmonary edema until they die of asphyxiation. Sulfur dioxide has a synergistic effect with soot in the atmosphere. When the concentration of sulfur dioxide in the atmosphere is 0.21 ppm, the soot concentration is greater than 0.3mg/L, which can increase the incidence of respiratory diseases and rapidly worsen the condition of patients with chronic diseases. Smoke events such as the London smog incident, the Maas Valley incident and the Donora incident are all hazards caused by this synergy.
First aid measures: Skin contact: Immediately remove contaminated clothing and rinse with plenty of running water. Medical treatment.
Eye contact: Lift the eyelids and rinse with running water or normal saline. Medical treatment.
Inhalation: Quickly leave the scene to a place where the air is fresh. Keep the airways open. If breathing is difficult, oxygen is given. If breathing stops, perform artificial respiration immediately. Medical treatment. [2]
In case of poisoning, the patient should be immediately moved to a place where fresh air is available, tight clothing should be undressed, oxygen should be inhaled quickly, the eyes and nasal cavity should be rinsed, and the mouth should be rinsed with 2% soda solution. If accidentally splashed in the eyes, rinse quickly with plenty of warm water. Severe cases should be sent to the hospital for treatment immediately