Source of this article: Time Weekly Author: He Mingjun
After two rounds of inquiries, Shanghai Haihe Pharmaceutical Research and Development Co., Ltd. (hereinafter referred to as "Haihe Pharmaceutical") was pressed to pause its journey to the Science and Technology Innovation Board.
On July 20, the review status of Haihe Drugs on the official website of the Shanghai Stock Exchange was changed to a suspension of review. According to the prospectus, Haihe Pharmaceutical intends to publicly issue no more than 221 million shares and raise 3.150 billion yuan for new drug research and development projects, Taizhou production base construction projects and supplementary working capital.
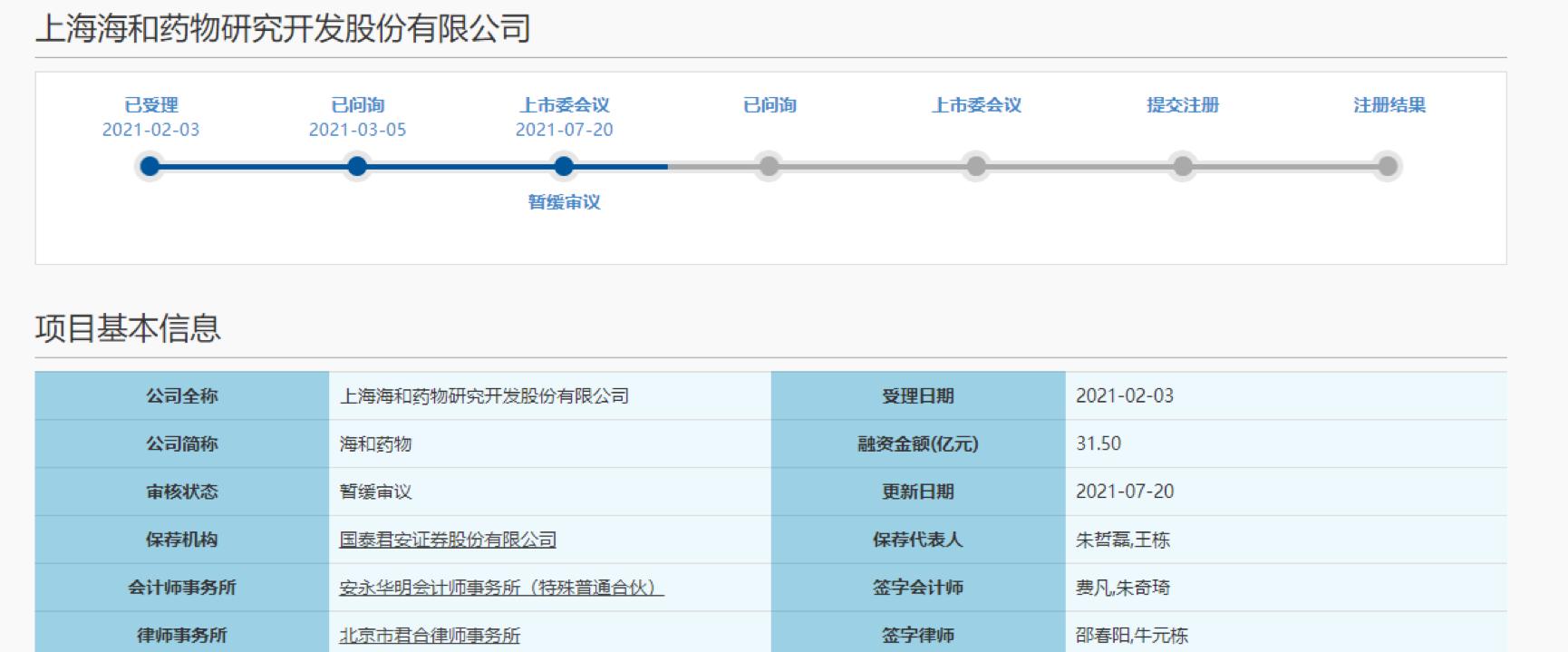
According to the Announcement of the Results of the 48th Review Meeting of the Listing Committee of the Science and Technology Innovation Board in 2021, Haihe Pharmaceutical was required to elaborate on issues such as core technologies, related party transactions, and equity transfers. At present, Haihe Drugs has not responded to this.
On July 30, in response to a number of issues disclosed in the announcement of the results of the review meeting, the Times Weekly reporter repeatedly called the secretary office of the board of directors of Haihe Drugs, but as of press time, the phone has not been connected.
Capital support, research and development capabilities are in doubt
Founded in 2011, Haihe Pharmaceutical is an independent innovation biotechnology company focusing on the discovery, development and commercialization of innovative anti-tumor drugs.
According to the data of Anxin International Research Report, the global oncology drug market has broad prospects, with the global market value of targeted therapy and immunotherapy reaching $125.8 billion in 2020, and is expected to increase to $433.3 billion in 2030. China's oncology market has grown rapidly in recent years, with a market value of $19.2 billion in 2016 and $30.4 billion in 2020, in line with an annual growth rate of 12.1%.
Aiming at the track of oncology drugs, Haihe Drugs has made many layouts. According to the prospectus, Haihe Pharmaceuticals conducts more than 15 clinical trials around 7 phases of clinical research on compounds. As of the date of signing of the prospectus, Haihe Pharmaceutical has focused on promoting 8 compounds in the field of anti-tumor, of which 7 compounds are in the clinical research stage and 1 compound is in the pre-clinical research stage.
The market value of oncology drugs has attracted the attention of many capitals. Tianyan's investigation shows that the investors of Haihe Pharmaceutical include Hillhouse Capital, CMB International, CICC Capital, Boyuan Capital and other well-known investment institutions. Warburg Pincus Capital, which holds more than 5% of the company's shares, said when leading the Series B financing of Haihe Pharmaceutical that Haihe Pharmaceutical's more leading products are expected to be approved for listing within two years, with greater commercial potential, while other products need relatively longer.
Haihe Pharmaceuticals' investment in research and development expenses has also increased year by year. According to the prospectus, from 2018 to 2020, the research and development expenses of Haihe Drugs were 106 million yuan, 303 million yuan and 482 million yuan, respectively. However, it is puzzling that Haihe Drugs, which focuses on the development of innovative drugs, has a much lower investment in research and development equipment than its peers.
According to the prospectus, the original value of Haihe pharmaceutical research and development equipment was 6.5533 million yuan, and the book value of depreciated research and development equipment was 2.6168 million yuan, and the equipment renewal rate was 39.93%. And Microchip Bio (688321. SH) and Zejing Pharmaceutical (688266.SH), the original value of the R&D equipment disclosed in their respective prospectuses is more than 10 million yuan.
Taking Microchip Biologics as an example, as of December 31, 2018, among the fixed assets of Microchip Biologics, the original book value of R&D equipment was 14.5849 million yuan, the net book value was 6.5311 million yuan, and the equipment renewal rate was 44.78%.
The Listing Committee of the Science and Technology Innovation Board questioned this in the first round of inquiry letter, requiring Haihe Drugs to explain the specific role of major R&D equipment on product research and development in combination with core technologies, and to explain the rationality of the book value of R&D equipment in combination with the situation in the same industry.
In its reply to the first round of inquiry letter, Haihe Pharmaceutical said that at this stage, the company's drug production is mainly carried out through entrusting CDMO agencies, and no large-scale purchase of drug production equipment has been purchased.
In addition, haihe drugs also have data fights in the number of R & D personnel.
The prospectus discloses that from 2017 to 2020, the number of employees of Haihe Pharmaceutical is 120, 96, 129 and 195 respectively. The national enterprise credit information publicity system shows that from 2017 to 2020, the total number of employees who paid pension insurance and medical insurance for Haihe Drugs was 93, 90, 1226 and 141 respectively.
(Image Source/Network)
In terms of products, the self-research ability of Haihe Drugs has also been questioned.
The Times Weekly reporter found that 8 of the 9 main product pipelines of Haihe Pharmaceutical were introduced or cooperatively developed. The project party with the most cooperative research and development is from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences (hereinafter referred to as the "Institute of Pharmaceuticals"), and Ding Jian, the founder and chairman of Haihe Pharmaceuticals, once served as the director of the institute.
According to the prospectus, the three core products of Haihe Pharmaceutical are RHX3001 oral paclitaxel, AL3810 delitinib, and SCC244 gumetinib. Among them, RMX3001 oral paclitaxel was introduced from Daehwa in South Korea in August 2017, and Haihe Drugs assisted in the clinical application of gastric cancer and breast cancer in China, and only has exclusive rights in Chinese mainland, Taiwan, Hong Kong and Thailand. AL3810 delitinib is also licensed to introduce, and Haihe Drugs has an exclusive interest in China. SCC244 Gumetinib is a joint venture between Haihe Pharmaceuticals and Pharmaceuticals, with global development interests.
The fifth set of listing standards of the Science and Technology Innovation Board stipulates that the expected market value of enterprises is not less than RMB 4 billion, the main business or products need to be approved by the relevant state departments, the market space is large, and phased results have been achieved. Enterprises in the pharmaceutical industry need to have at least one core product approved to carry out phase II clinical trials, and other enterprises that meet the positioning of the science and technology innovation board need to have obvious technical advantages and meet the corresponding conditions.
Due to the excessive introduction and cooperative research and development technology, the Listing Committee of the Science and Technology Innovation Board requested Haihe Pharmaceutical to explain whether there is a significant dependence on third-party technology mainly relying on the authorization to introduce or cooperative research and development to introduce related product pipelines, and at the same time, combined with the fact that the core products that have carried out more than phase II clinical trials are derived from authorized introduction or cooperative research and development, whether they meet the fifth set of listing standards stipulated in the "Review Rules for the Issuance and Listing of Shares on the Science and Technology Innovation Board of the Shanghai Stock Exchange".
"The Listing Committee's request to self-check whether it meets the listing criteria shows that the Listing Committee has doubts about the certainty of the core knowledge products that support the operation of Haihe Drugs, and further explains and explains the details and terms of the authorized introduction to ensure the exclusivity and continuity of the enterprise for the intellectual property rights." On July 31, IPG China chief economist Bai Wenxi said in an interview with the Times Weekly reporter.
"If this enterprise cannot solve the problem of certainty of core intellectual property rights, it will inevitably affect the independence and sustainable operation of the enterprise, and naturally it will not meet the listing standards." Bo Wenxi added.
Academician platform, the "story king" figure flashed
The reason why Haihe Pharmaceutical can get the favor of capital and become a star enterprise, in addition to being in a good track, is also closely related to the background of the founder team.
Ding Jian, chairman of Haihe Pharmaceutical, is an academician of the Chinese Academy of Engineering. According to the prospectus, Ding Jian served as the director of the Institute of Pharmacy from 2004 to 2013 and is now the leader of the research group of the Pharmacology Room of the Institute of Pharmacology. Ruiping DONG, general manager of Haihe Pharmaceutical, has many years of experience in the research and development and management of international pharmaceutical giants.
In March 2011, the Institute of Pharmaceuticals and Zhangjiang Ketou established a joint venture between Haihe Pharmaceutical. In December 2013, Zhangjiang Ketou transferred all 50% of its equity in Haihe Pharmaceutical to Zehao Investment for 59 million yuan. The Wang Weilin family, the "story king" of A shares, is the actual controller of Zehao Investment.
In 2014, Ze Hao invested in "left hand to right hand" and transferred 50% of the equity to Tibet Nanjiang Investment Co., Ltd. (hereinafter referred to as "Tibet Nanjiang") also under the control of the Wang Weilin family for 60 million yuan.
After the completion of the above-mentioned equity transfer, the major shareholders of Haihe Pharmaceutical were changed to the pharmaceutical institute and the Wang Weilin family.
In July 2016, the Pharmaceutical Institute transferred 19.51% of its shares in Haihe Pharmaceutical through public listing, and Green Valley Group, which started as a health product, acquired it at a price of 80.1895 million yuan. A month later, Green Valley Group transferred its 19.51% stake to Nanjiang, Tibet as compensation for breach of contract. So far, Tibet Nanjiang has held a total of 69.51% of the equity of Haihe Pharmaceutical.
Subsequently, Tibet Nanjiang decided to carry out a total of not less than 55% but not more than 60% of the equity incentives for Ding Jian (one-time transfer of 40% of the equity and 15%-20% option incentives), and transferred 40% of the equity to Ding Jian at a one-time price of 1 yuan, of which 19.5122% of the equity came from Green Valley Group and 20.4878% from Tibet Nanjiang. In addition, 16.48% of the equity is given to Ding Jian in the form of options.
After a series of operations, Ding Jian personally changed from zero shareholding to a major shareholder of Haihe Pharmaceutical, but the equity transfer price of 1 yuan caused questions from the Listing Committee of the Science and Technology Innovation Board, asking Haihe Pharmaceutical to explain the relevant situation of the transfer of state-owned assets.
According to the prospectus, as of the date of signing of the prospectus, Ding Jian directly held 22.7640% of the equity of Haihe Pharmaceutical, and indirectly controlled 6.6456% of the equity of Haihe Pharmaceutical through the employee shareholding platform of Haihe Pharmaceutical - Shanghai Heying, and Ding Jian directly and indirectly owned 29.4096% of the voting rights of Haihe Pharmaceutical, which is the controlling shareholder and actual controller of Haihe Pharmaceutical.
It is worth mentioning that the Green Valley Group, which acquired the equity of Haihe Pharmaceutical at a high price, is actually quite close to Ding Jian.
In January 2008, CCTV broadcast a report exposing the "Green Valley" scam, reporting: "Since 1996, Green Valley Group has launched three generations of so-called anti-cancer products, namely China Lingzhi Bao, Shuangling Gu Benshan and Green Valley Lingzhi Bao. Green Valley Group has continuously changed its name for more than 10 years and published product advertisements without approval, becoming one of the most typical series of false advertising cases in the country in the past ten years. ”
The Times Weekly reporter noted that in 2004, Professor Chen Jinsheng, a shareholder of Green Valley Group, said in his paper "Research and Clinical Application of Shuangling Solid Benshan Anti-tumor" that the inhibition rate of Shuangling Solid Benshan on liver cancer cells was as high as 93.6%, and the suppression rate of lung cancer was as high as 100%. At that time, Green Valley Group claimed that this experimental data was obtained in cooperation with Professor Ding Jian of the Institute of Pharmaceuticals.
According to Haihe Pharmaceutical's reply to the first round of inquiry letters, Ding Jian served as a director of Green Valley Pharmaceutical, a subsidiary of Green Valley Group, from December 2002 to February 2020.
The Wang Weilin family, the actual controller of the Nanjiang River in Tibet, is a famous "story king" in the A-share market, and the hype theme is its best play.
Nanjiang, a Shanghai nanjiang controlled by the Wang Weilin family, is the majority shareholder of the A-share listed company Huali Family (600503.SH), which has borrowed Xu Xiang's hand to push up the stock price and profit from it through the mode of "stripping real estate" and speculating on hot topics, including graphene, intelligent robots, financial futures, biomedicine and other concepts that have been speculated.
Some market views have pointed out that in recent years, it is very common to find capital to pay, find scientists to stand, and buy products under development from overseas pharmaceutical companies to set up a platform and outsource to contract research organizations (CROs). "21st Century Business Herald" also reported that Haihe Pharmaceutical is a typical capital saving model, piecing together the image of an innovative pharmaceutical company in the form of "License in", and then securitizing it and realizing it through the capital market.
However, the "License in" model is purely marketing-driven and does not have the ability to develop independently in the true sense. Pharmaceutical companies that lack the attributes of science and technology innovation are naturally difficult to meet the listing standards of the science and technology innovation board.
Companies that have been suspended after two rounds of inquiries are rare in the history of IPOs on the Star Market.
Since the beginning of this year, Fujian Huichuan Internet of Things Technology Co., Ltd. and Suzhou Linhua Medical Device Co., Ltd. have also been suspended for consideration at the first meeting, and the meeting has not been passed again, and has been terminated by the Listing Committee of the Science and Technology Innovation Board. It is worth mentioning that the above two companies, like Haihe Pharmaceutical, have hired Guotai Junan as the lead underwriter and sponsor of the brokerage.