On January 6, the U.S. Food and Drug Administration (FDA) approved Leqembi (lecanemab-irmb) for the treatment of Alzheimer's disease (AD) through an accelerated approval pathway. Leqembi is the world's first AD drug clinically proven to slow memory and thinking decline by 27% after taking the drug for 1 and a half years, helping people in the early stages of AD maintain their minds. Eisai and Biogen partnered to develop Leqembi and priced it at $26,500/year. The pricing regulator (ICER) said the drug was priced between $8,500 and $20,600 "cost-effective" and above that price would be "difficult to meet cost-effectiveness thresholds."
Leqembi: Non-curative drug that removes amyloid plaques in the brain early in AD
Although the specific cause of AD is not fully understood, it is roughly the failure of brain proteins to function properly, thereby disrupting the function of brain cells (neurons), triggering a series of toxic events, resulting in neuronal damage, loss of connection to each other, and eventual death. This type of damage usually begins in the region of the brain responsible for controlling memory, but this process begins years before the initial symptoms appear; Subsequently, neuronal loss spreads to other areas of the brain in some predictable pattern; Finally, by the end of the disease, the brain has shrunk significantly. Therefore, in the early stages of AD, it is extremely important to stop brain protein abnormalities and reduce amyloid plaques for the treatment of AD.
Leqembi is a humanized immunoglobulin G1 (IgG1) anti-amyloid β (Aβ) monoclonal antibody that selectively binds to neutralize and remove soluble toxic amyloid aggregates β Although many previous drugs targeting amyloid have failed to slow the rate of mental loss in patients, clinical studies have shown that intravenous administration of Leqembi every other week can remove amyloid from the brain. Based on the results of Leqembi's randomized controlled trial (Study 201) of amyloid plaque burden reduction measured by PET imaging, the FDA decided to approve the drug for the treatment of AD under an accelerated approval pathway.
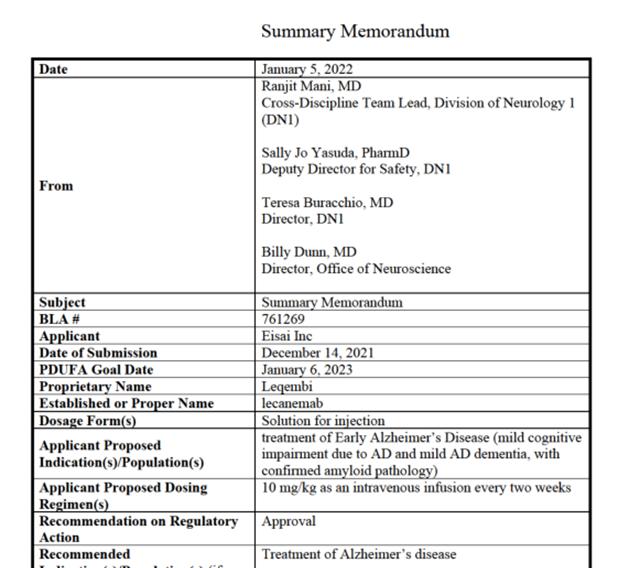
Figure 1 Summary review of Leqembi released by the FDA Center for Drug Evaluation and Research (Source: [1])
01
Leqembi met all key clinical secondary endpoints
Study 201 was a multicenter, randomized, double-blind, placebo-controlled, parallel-group study of 856 patients with mild cognitive impairment (MCI) due to AD or mild AD dementia. Changes in cerebral amyloid plaques were measured by PET and assessed at weeks 53 and 79 by composite standard uptake value ratio (SUVR) for cerebral amyloid plaque changes in a subset of patients and as endpoints in support of accelerated approval.
Leqembi was found to reduce brain amyloid plaques in a dose- and time-dependent manner, meeting all key secondary endpoints. The Leqembi group using 10 mg/kg every two weeks had a statistically significant reduction in brain amyloid plaques from baseline to week 79 compared with placebo. Preliminary analysis of the AD composite score at week 53 showed that the biweekly dosing regimen of Leqembi 10 mg/kg (64%) was superior to the placebo group (25%). Data at week 79 showed a reduction of about 20 to 40 percent in the decline in clinical endpoints.
02
Leqembi has side effects
The most common side effects of Leqembi are amyloid-related imaging abnormalities (ARIA), headache, and infusion-related reactions. ARIA most commonly presents with temporary swelling and effusion in brain areas (ARIA-E) and may be accompanied by small bleeding spots inside or on the surface of the brain and superficial siderosis (ARIA-H), and some people may experience symptoms such as headache, confusion, dizziness, decreased vision, nausea, and seizures. Infusion-related reactions include flu-like symptoms, nausea, vomiting, and changes in blood pressure.
In the 10 mg/kg Leqembi treatment group every two weeks, the incidence of ARIA-E was 10% and the infusion response was 20%, compared with 1% and the infusion response was 3% in the placebo treatment group. The most common symptoms of participants treated with Leqembi 10 mg/kg were headache, confusion/altered mental status, agitation, and visual disturbances. Leqembi infusion reactions occurred in 88% of the first infusion, with a mild incidence of 56% and moderate incidence of 44%, and symptoms included fever and flu-like symptoms (chills, general pain, feeling of trembling, and arthralgia). There were no fatalities in Study 201.
Leqembi Phase 3 clinical trial: delayed 27% of memory and thinking decline, but 3 people died
On January 5, NEJM released the results of the Leqembi global Phase 3 clinical trial (Clarity AD) [2]. Clarity AD is an 18-month global multicenter, placebo-controlled, double-blind, parallel, open-ended, Phase 3 clinical trial to evaluate the efficacy and safety of Leqembi in the treatment of early-stage AD. 235 research centers in Japan, the United States, Europe, China, South Korea, Canada, Australia and Singapore enrolled 1,795 patients with early-stage AD. It is worth expecting that China also participated in the phase 3 clinical trial of the drug, collecting a total of 111 patients, due to the late start of the Chinese study, the clinical trial is still ongoing, and the Chinese patient results are expected to be announced in the second half of 2023 to reveal whether there are differences in efficacy between different races [3].
Figure 2 Results of Leqembi's global phase 3 clinical trial (Source: [2])
In the Clarity AD clinical trial, patients with early-stage AD received intravenous Leqembi or placebo every two weeks for 18 months, and researchers used dementia cognitive rating scales such as CDR-SB, ADAS-cog, ADCOMS and ADCS-MCI-ADL to obtain significant scores in the medication group than in the control group, suggesting that Leqembi significantly improved cognitive function. It also suggested that patients who took the drug were significantly less likely to progress to the next stage of the disease (31%), and 27% of patients delayed memory and thinking loss symptoms for a year and a half. However, the incidence of ARIA-E was 12.6% in the Leqembi group and 1.7% in the placebo group. For ARIA-H or signs of bleeding, the incidence was 17.3% and 9.0%, respectively.
There were 3 deaths in the Phase 3 trial: the first was a patient with atrial fibrillation who was taking the blood thinners apixaban (Eliquis); The second was a 65-year-old participant who received a tissue plasminogen activator (tPA) for acute stroke, where the combination of t-PA and Leqembi may have triggered a fatal cerebral hemorrhage and bleeding cascade; The third case, a 79-year-old participant, developed extensive brain swelling, hemorrhage, and seizures.
Rudolph Castellani, a neuropathologist at Northwestern University, made Leqembi's role in patient death more explicit in an article published in Science in November. Castellani said: "In my opinion, this is a disease and death caused by Leqembi treatment, and I have no doubt about that. ”
Marwan Sabbagh and Christopher van Dyck, who conducted the clinical trial, believe there is not enough evidence to blame Leqembi for the death. In their accompanying reply letter to NEJM, they said: "Treatment with t-PA alone can also cause fatal bleeding. Earlier it was reported that t-PA treatment of people with cerebral amyloid angiopathy can cause fatal catastrophic intracerebral hemorrhages in the absence of any anti-amyloid drugs. ”
In response to the deaths, the doctors said: "We would use Leqembi with caution in patients who use certain blood thinners and carry the APOE4 gene because these patients have a higher risk of side effects." However, we would prescribe Leqembi to AD patients instead of Aduhelm because there is more evidence that Leqembi is effective. ”
Leqembi is priced at $26,500/year, which ICER believes is too high
Eisai and Biogen eventually priced Leqembi at $26,500/year. Eisai said the "annual price" previously calculated based on Phase II results ranged from $9,200 to $35,600, which rose to $37,000 after considering Phase III data as part of the "social value of the drug." However, considering that the previously launched similar drug Aduhelm had poor sales due to cost and efficacy issues, and that the federal health insurance (CMS) limited coverage of anti-amyloid drugs, patients over the age of 65 must participate in clinical trials to receive health insurance services, Leqembi's pricing was eventually reduced to $26,500/year.
Pharmaceutical analysts see Aduhelm and Leqembi pricing as similar, with investors expecting close proximity to Biogen and Eisai's $28,000/year pricing for Aduhelm. Most new drugs are under scrutiny in terms of pricing, but Leqembi has received special attention because no other drug can change the course of AD. Aduhelm was initially priced at $56,000/year, but its annual price was eventually cut to $28,200 due to the debate over Aduhelm's clinical significance, heightened safety concerns stemming from patient death reports, and the likely large number of patients. Despite the price adjustment, Aduhelm sales are still poor compared to the billions of dollars in sales Biogen once expected.
Pricing regulator ICER said that given the large number of AD patients, it is particularly important for Leqembi to align its pricing with its value to patients. Leqembi is priced between $8,500 and $20,600 "cost-effective," and pricing above that "won't meet typical cost-benefit thresholds."
"Leqembi should be available now, but without health insurance, only the wealthy who can afford $26,500 a year can access this treatment, making it inaccessible to millions of AD patients," Us Against Alzheimer said. Leqembi's pricing should be fair and appropriate, so that every AD patient can benefit from it. ”
Lars Lannfelt, the inventor of Leqembi and an Eisai partner, called Aduhelm's pricing a "mistake" in an interview, but he was reluctant to comment on Leqembi's price, saying only that every AD patient should benefit from Leqembi.
Written by | competition for essays
Typesetting| Competition for Text
End
Resources:
[1]https://www.accessdata.fda.gov/drugsatfda_docs/summary_review/2023/761269Orig1s000SumR.pdf
[2]van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer's Disease. N Engl J Med. 2023 Jan 5;388(1):9-21. doi: 10.1056/NEJMoa2212948. Epub 2022 Nov 29. PMID: 36449413.
[3] JIA Jianping, QUAN Meina. Reflections on the results of another new drug phase 3 clinical trial for Alzheimer's disease based on the Aβ theory.
This article is original by BioExploration, and individuals are welcome to forward and share. If any other media or website needs to be reproduced, the source of biological exploration must be indicated before the text.