In late May 2021, British biotech company Immunocore announced the first slow hepatitis B subject administration in the IMC-I109V Phase 1 clinical trial in the hepatitis B drug development pipeline, and since 2022, the company has continued to update the development progress of this new hepatitis B drug under research.
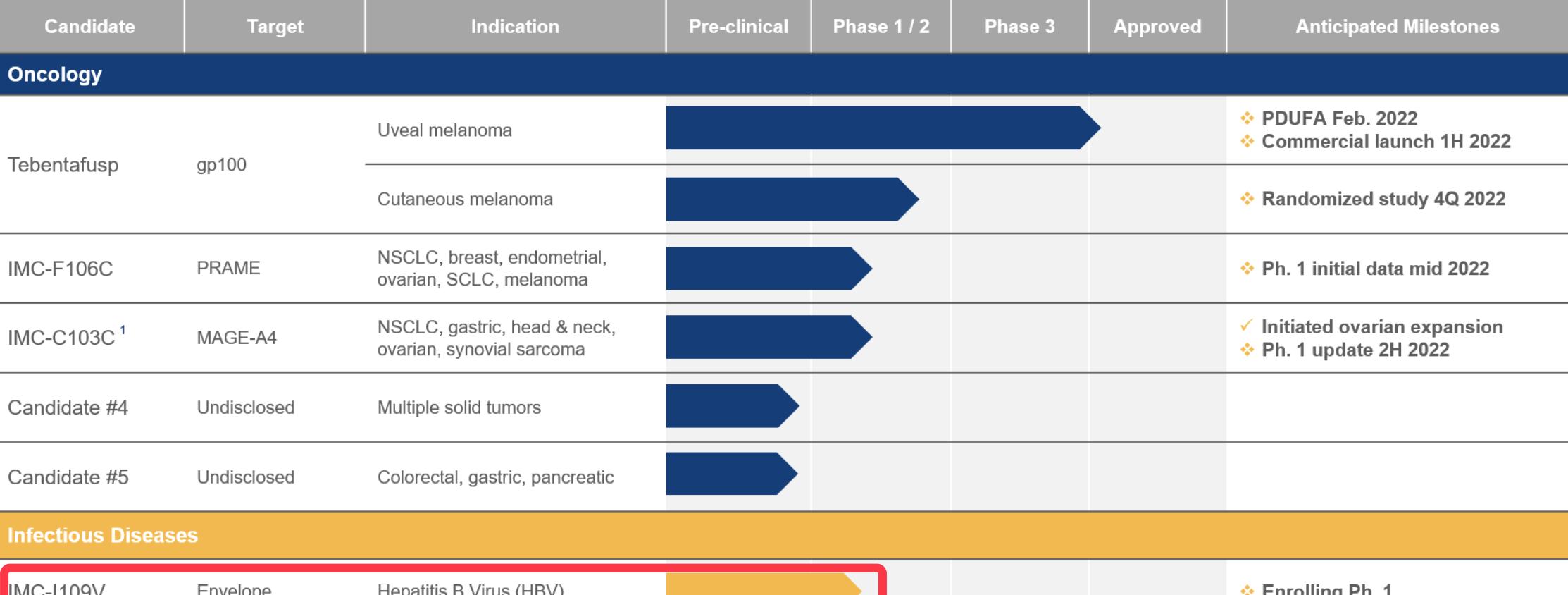
Pipeline from Immunocore: Visible red notes for IMC-I109V continue in Issue 1
Two new drugs for hepatitis B were renewed, AB836 was enrolled in Phase 1, Part 3, and IMC-I109V continued in Phase 1
Immunocore introduced that it will continue to include subjects in the IMC-I109V global Phase 1 escalation dose trial in 2021. IMC-I109V is a hepatitis B drug candidate developed using an antiviral immune T cell monoclonal antibody (ImmTAV®) platform and entered clinical trials.
IMC-I109V targets a conservative hepatitis B virus (HBV) envelope antigen and is being developed as a potential functional cure. Currently, the IMC-I109V, which targets HBV, is still in Phase 1 clinical trials (the above progress is published in Immunocore's latest pipeline update).
Yesterday, Arbutus' hepatitis B new drug under development by Yangmei Biopharmaceutical Company only introduced the progress of AB-729, and there are three pipeline drug candidates today together. AB-836 is an investigational oral HBV capsid inhibitor, introduced in Arbutus's latest pipeline, it is the next generation of new oral capsid inhibitors developed by Arbutus, with higher intrinsic potency, anti-resistance variant activity and enhanced cccDNA replenishment capacity, which is the reason for the persistence of HBV.
Currently, Arbutus is being included in the third part of the ongoing phase 1a/1b clinical trial in which subjects are enrolled in the ongoing AB-836 phase 1a/1b trial evaluating the safety and tolerability of multiple doses of AB-836 in cHBV-infected patients. More data from cHBV-infected patients is expected to be reported in the first half of 2022.
In addition, readers are more concerned about Arbutus' newly developed preclinical phase compounds AB-101 and AB-161. AB-101 is an oral PD-L1 inhibitor under investigation designed to reawaken the immune system of chronic hepatitis B patients, which Arbutus believes may be a key component in developing a functional cure for HBV. Arbutus has begun IND studies (New Drug Clinical Trial Applications) for AB-101 and intends to complete these studies in the second half of 2022.
AB-161 is a research-under-research oral RNA destabilizer, which is also the next generation of oral HBV-specific RNA destabilizer developed by Arbutus and is being developed as a full oral treatment regimen aimed at achieving a functional cure for HBV. Arbutus conducted an extensive non-clinical safety assessment of AB-161 to avoid the risk of developing ab-452, a first-generation oral RNA destabilizer that had failed to develop (due to peripheral neuropathy found in non-clinical safety studies). Arbutus has conducted IND studies on AB-161 and intends to complete them in the second half of 2022.
William Collier, President and CEO of Arbutus, commented: "We have established a strategic and clinical partnership that allows us to explore several combination therapies with AB-729, an RNAi therapeutic agent in the hepatitis B drug pipeline, as a cornerstone drug for a potential functional cure for hepatitis B, and expand the coverage of AB-729 to the Greater China region.
In addition, we have expanded our pipeline for new hepatitis B drug development, with two preclinical programs including the oral PD-L1 inhibitor AB-101 and the oral RNA destabilizer AB-161, both of which are expected to complete in-drug licensed studies this year (completing Phase 1 clinical trials!). )。 AB-729 and AB-836 are expected to publish several key clinical trial data later this year that will inform advancing them into Phase 2b clinical development.
Xiaofan Health Conclusion: Because Arbutus's hepatitis B new drug pipeline is more updated, therefore, AB-729 (yesterday has been popularized, but also the pipeline progress is faster) and AB-836, AB-101, AB-161 in two days. Phase 1 and Phase 2A clinical trials of AB-729 are underway at the same time, and multiple combination therapies, including other innovative drugs under investigation, are underway.
AB-836 is an HBV capsid inhibitor under research, and we are already familiar with this drug target; Arbutus' latest highlight is AB-101 and AB-161, whose chemical structure, mechanism of action and life cycle steps of hepatitis B virus are different from AB-729 and AB-836, and AB-101 and AB-161 are expected to complete new drug clinical trial applications in the second half of this year, that is, IND research!