The gospel for patients with dry macular degeneration is coming.
Recently, Shenzhen Eglin Pharmaceutical Co., Ltd. (hereinafter referred to as "Eglin Pharmaceutical") announced that the company has independent intellectual property rights, oral drug EG-301 for the treatment of dry macular degeneration, and its IND application has been approved by the US FDA and officially entered the second phase of clinical trials. It is reported that this is the world's first phase II ophthalmic clinical study on this target to confirm the clinical efficacy of EG-301 on Dry AMD.
Eglin Pharmaceutical is an innovative pharmaceutical company with artificial intelligence (AI) as the main core technology. According to reports, EG-301 is an oral drug used to treat dry macular degeneration over 50 years old, and there are currently complete human safety data and its effectiveness has been proved in animal experiments.
Dry macular degeneration is a type of age-related Macular Degeneration (the other is wet), AMD is a chronic and irreversible eye disease that occurs more often in people over the age of 50, causing damage to the eye cells in it, death and leading to vision loss. According to statistics, there are about 100 million people with macular degeneration worldwide in 2020, of which dry AMD accounts for about 90% of the total.
However, there is currently no specific drug for the treatment of dry macular degeneration, and can only delay the disease by allowing patients to take vitamins and zinc, which can be said to be "an untapped virgin land with unlimited market potential"; wet macular degeneration can improve symptoms through anti-VEGF drugs and surgical treatment to reduce angiogenesis.
It is worth noting that EG-301 is based on Eglin Pharma's own, AI-driven drug mechanism evaluation platform, which has conducted in-depth discussion and prediction of EG-301's new targets, pathogenesis, intraocular efficacy, lysosomal drug generation and development risks, showing that EG-301 enhances the anti-inflammatory and antioxidant functions of retinal pigment epithelial cells, improves epithelial cell autophagosome transport function, inhibits epithelial cell complement activation, and has the effect of protecting mitochondria.
What has been proven by the industry is that AI has a significant advantage in improving the efficiency of new drug development. With this as a line of thinking, Eglin Pharmaceutical has built a drug innovation platform in the United States with AI and drug research as the core. The platform is mainly used to support the in-depth application of AI technology in the design and screening of new drugs, the development and optimization of new formulations, and drug development.
According to Dr. Du Tao, co-founder and chairman of Eglin Pharmaceuticals, unlike the AI technology platform of third-party companies on the market, which mainly focuses on the preclinical stage such as target discovery and chemical synthesis, the company has applied its own AI in the development stage, including regulatory toxicological research, pharmacokinetics and clinical trial design.
However, this is only one part of Eglin Pharma's layout of "efficient research and development". Previously, Eglin Pharmaceutical also developed innovative drugs with the idea of "old drugs and new uses". A typical result is that less than 2 years after its establishment, the company has had an innovative drug candidate EG-007 approved by the US FDA to carry out phase III key clinical trials with targeted drugs and anti-PD-1 antibody drugs for the treatment of advanced endometrial cancer. The main goal of this clinical trial is to improve the response rate/efficiency of immunotherapy in patients with advanced endometrial cancer by combining with EG-007.
36Kr learned during the interview that Dr. Du Tao has rich experience in the medical field, and he has worked as a new drug review officer in the US FDA for 7 years, and then served as an executive in a large CRO and international pharmaceutical company, and has also engaged in pharmaceutical investment and pharmaceutical development and regulatory related consulting, these professional resumes have given him rich experience and insight into whether it is capital markets, innovative development, or new drug declaration.
The reform of the Food and Drug Administration in 2015 and the 18A New Deal launched by the Hong Kong Stock Exchange in 2018 have made innovative drugs a "fragrant feast" in the eyes of capital and entrepreneurs. After several years of wait-and-see and inspection, Du Tao also believes that the current industrial situation has been "unprecedented prosperity", "talents, capital, technology and other elements are available, to achieve the goal of making up for the gap between the Pharmaceutical industry between China and the United States, it is time to do it", so in 2019 officially founded Eglin Pharmaceutical.
Different from the general innovative drug companies chasing hot targets (such as piling up PD-1) or large indications (such as betting on tumor tracks), Eglin Pharmaceutical has formulated four development strategies: focusing on clinical needs, facing the international market, efficient development, rapidly entering clinical trials, and locking in the two major areas of immunology (including tumor immunology) and ophthalmic drugs.
The so-called focus on clinical needs, Dr. Du Tao and the team have locked it into the first-line drug field of "so far there has been no drug approved", which can also be popularly understood as "no drug can be treated". This can be seen in Eglin's current layout of concern.
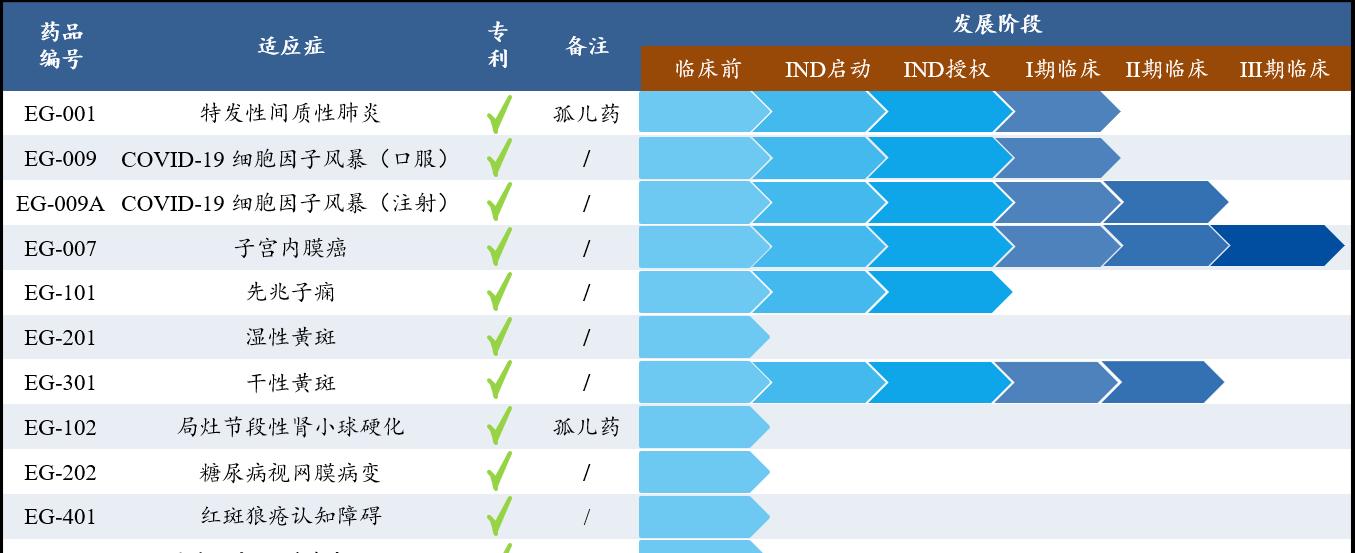
Eglin Pharma R&D pipeline
Taking EG-007, which treats endometrial cancer, as an example, although immunotherapy drugs account for more than 60% of clinical trials of endometrial cancer, they still generally face the problem of low response rates to immune checkpoint drugs. It is reported that animal experiments have shown that EG-007 can enhance the therapeutic effect of anti-PD-1/L1 antibodies, and the combination of EG-007 and immunotherapy is expected to become a first-line therapy for the treatment of advanced endometrial cancer.
Another example is the company's self-developed EG-009A for the new crown cytokine storm. According to Dr. Du Tao, the cytokines secreted by human white blood cells are one of the important weapons against viruses, but sometimes they will "get out of control" and attack their own organs and tissues. In the middle stage of the new crown virus infection, the virus has generally stopped replicating, and moderate to severe patients have an overactive immune response, which may endanger the patient's own "cytokine storm", which EG-009A can inhibit, thereby treating moderate to severe new crown pneumonia.
At present, in the world, there is not a single "cytokine storm" caused by the treatment of new crown pneumonia in the current approved drugs, including the US EUA. Currently, EG-009A is available in two dosage forms, oral and intramuscular, which can be used separately or in combination. If you give a fast-acting injection first, quickly increase the blood drug concentration, and then use oral agents to maintain the blood drug concentration for the convenience of patients.
In addition, Dr. Du Tao introduced that the pipelines for idiopathic interstitial pneumonia, pre-eclampsia, dry macula, Crohn's and other pipelines are also in line with the status quo of "no drug to treat", even if wet macula has medicines to treat, but the current intraocular injection experience is also very bad, seriously affecting patient compliance.
As for facing the international market, this also coincides with the current trend of major innovative pharmaceutical companies going overseas. Dr. Du Tao introduced that limited by the current domestic medical insurance payment system, the pricing of innovative drugs is bound to be constrained, and it is easy to lose under the trend of normalization of collection and procurement, and if there is a global business layout, these problems will be solved. Therefore, Eglin Pharmaceutical has developed an international R&D and marketing strategy from the beginning of its establishment, and has taken the lead in launching the corresponding filing procedures and clinical trials in the US FDA.
Dr. Du Tao believes that in the process of seizing the international market, efficient development is particularly important, "which can directly determine whether you are a first-line drug or a second-line drug in many diseases."
How to achieve efficient development and clean and sharp, in addition to the use of AI technology to quickly discover new molecules, he believes that there are two main points: first of all, based on the strategy of "high supervision" market, to ensure that it can be fully recognized in the subsequent registration approval process, to achieve rapid approval, "take Hong Kong as an example, if a drug is approved in the United States or major European countries, its registration declaration in Hong Kong will be very simple, about a few weeks to go public"; secondly, to be well versed in policies and regulations, to ensure that there is no detour. It is reported that the current Eglin team has 5 former FDA review officers, and the accumulation of knowledge in regulations can support the team to move forward quickly under the "high regulation" route.
In the process of studying old drugs, the Eglin pharmaceutical team has discovered several new targets related to important diseases. For these targets, with the help of the application of AI technology, a completely new drug molecule has been synthesized, "and these new drug molecules have higher activity and lower toxicity than the old drug molecules", and the aforementioned EG-301 is a typical result of this idea.
On this basis, Dr. Du Tao believes that it is necessary to quickly promote the clinic, "no matter how perfect the laboratory research, it is possible to fall short in the follow-up clinical research, and it is easy to waste several years, and the rapid promotion of the clinic can save time and reduce the cost of follow-up silence." It is precisely because of this kind of action that Eglin has a number of drugs entering the clinic and cooperating with major research institutions around the world to jointly develop them, in the same echelon of innovative pharmaceutical companies, the speed is amazing."
He said, "This era is the era of rapid development of China's pharmaceutical industry and the world, and I hope to live up to the era in which I am fortunate to live up to it, do something for China's pharmaceutical industry, think along the clinical value chain, and continue to make breakthroughs in the unsatisfied treatment field." ”
36Kr also understands that Eglin Pharmaceutical has now completed Pre-A and A rounds of financing and is currently in the process of conducting a new round of financing to meet the funding needs of new drug research and development and clinical trials.