This article is from the WeChat public account: X-MOLNews
Today, the precise assembly of various functional nanostructures and molecular devices can be basically achieved through modern nucleic acid and peptide nanotechnology. Recently, a scientific research team led by Lou Chenguang of the University of Southern Denmark and Mao Hanbin of Kent State University reported a powerful molecular experimental platform that organically fused nucleic acid polypeptide nanotechnology and optical tweezer single molecule force spectrometry, revealing for the first time that remote chiral action exists in two important types of biological macromolecules - nucleic acids and polypeptides of higher structures. The cross-cutting study was carried out by an international research team consisting of the University of Southern Denmark (Denmark), Kent State University (USA), the University of Copenhagen (Denmark), the University of Oxford (UK) and ATDBio (UK).
Chiral transmission often exists at close range between small molecules through intermolecular forces, but as a completely new force, whether chiral transmission is independent of intermolecular forces exists in nucleic acids and proteins of higher structures remains to be explored. General intermolecular forces such as hydrogen bonds, π accumulation, and hydrophobic action are chemical forces, while chiral transmission exists through physical mechanical forces. Its force is weak and relies on the chiral spiral structure of nucleic acids and proteins, and common methods cannot build sensitive experimental systems to detect this force. Therefore, how to detect weak chiral transmission signals in the background of other chemical forces with high decibels is a huge scientific challenge. The team pointed out that although chiral transmission is much weaker than the general intermolecular force, important biological macromolecules such as nucleic acids, proteins and chiral spirals inside polysaccharides are all single chiral, and small differences in chiral transmission may be one of the origins of our current biological macromolecule chiral world after a long history of earth evolution.
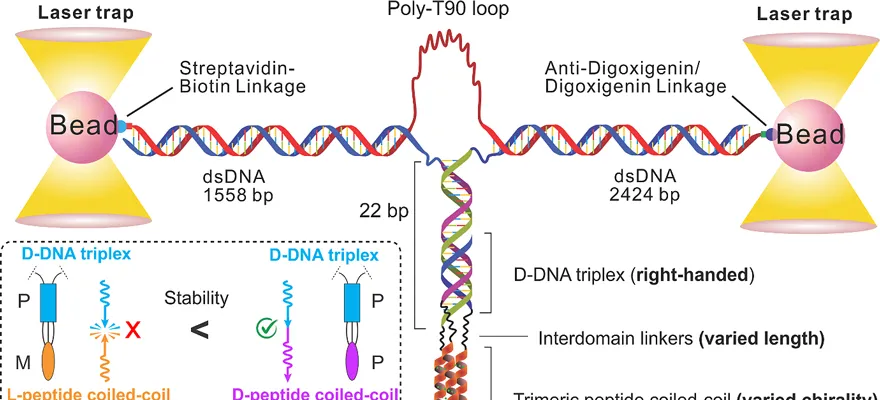
Figure 1. Schematic of phototunes for single-molecule analysis and chiral compatibility/incompatibility effects between two higher domains of nucleic acid and polypeptide
The work of Lou Chenguang and Mao Hanbin's team at the University of Southern Denmark and Kent State University fills this technical and cognitive gap. By organically combining nucleic acid peptide nanotechnology and optical tweezers single-molecule force spectroscopy, the team constructed a series of nucleic acid peptide tertiary structures at the molecular level, using the high sensitivity of single-molecule force spectroscopy to reveal for the first time that chiral transport forces exist in the advanced structure of nucleic acids and polypeptides (Figure 1). The study uses chemical means to mirror the tertiary structure of the polypeptide, retain the general chemical force between molecules, and use the higher structure of nucleic acid as a molecular probe to detect small differences in chiral transmission. The experimental results confirm that chiral forces do exist in the advanced domains of important biological macromolecules such as nucleic acids and proteins, and their intensity and distance between the two polymer domains are inversely proportional to those in the 4.5 nm range.
The study explains an interesting natural phenomenon as to why high-level domains of opposite chirality are rarely observed in the same protein molecule in native proteins. The answer is actually very simple, it is energy, because maintaining the opposite chirality requires higher energy, and nature has always sought as low energy channels as possible in the long process of evolution. The work was recently published in Nature Communications. Shankar Pandey, Shankar Mandal and Mathias Bogetoft Danielsen, PhD students from the University of Southern Denmark and Kent State University, were the first authors, and Associate Professor Chenguang Lou of the University of Southern Denmark and Professor Hanbin Mao of Kent State University were the corresponding authors. The research team would like to thank Associate Professor Peter W. Thulstrup, Dr. Josephine Tuborg Boesen, Dr. Kasper K. Sørensen, and Joan Hansen and Tina Grubbe Hansen of the University of Southern Denmark for their help with this work, as well as Villum Fonden (DK), National Institutes of Health (DK) USA), the National Science Foundation (USA) and other programs provide funding for this work.
Chirality transmission in macromolecular domains
Shankar Pandey, Shankar Mandal, Mathias Bogetoft Danielsen, Asha Brown, Changpeng Hu, Niels Johan Christensen, Alina Vitaliyivna Kulakova, Shixi Song, Tom Brown, Knud J. Jensen, Jesper Wengel, Chenguang Lou &Hanbin Mao
Common Nat., 2022, 13, 76, DOI: 10.1038/s41467-021-27708-4