Southern Finance and Economics All-Media Information Communication researcher Cui Haihua comprehensively reported that the innovative drug sector closed down in 2021. Against the backdrop of a 2021 correction of -11.57% in the biomedical sector (931152.CSI), the innovative drug subdivision closed down 6.54%.
Differentiation of the performance of innovative drug labels in 2021. At present, there are more than 40 innovative drug targets listed on the Hong Kong Stock Exchange and A-shares.
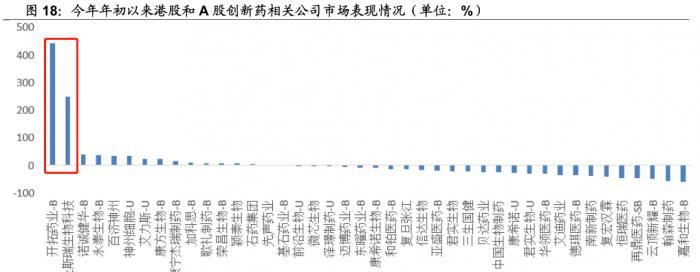
From the perspective of market performance, the market performance of the company represented by the Hong Kong stock BIOTECH this year is better, and the development of pharmaceuticals and Jinsrui Biotechnology has risen by more than 200% throughout the year, and some have also fallen by more than 40%.
The time for innovative drugs to enter medical insurance continues to shorten. The results of the 2021 annual medical insurance catalogue negotiations were released on December 3, and a total of 7 generic drugs directly entered the medical insurance and 67 new products were negotiated and transferred, of which 27 innovative drugs entered the medical insurance in the year of listing. The "seamless connection" between drug review and medical insurance review has been basically realized.
In 2021, the medical insurance negotiations will be favorable for the release of innovative drugs. In past health insurance negotiations, the vast majority of newly included and newly covered products have achieved very significant volume and sales growth.
According to public data, the sales of drugs that were negotiated to enter the Medicare list in 2017-2019 increased by 128%, 337% and 39% respectively compared with the year before inclusion.
SPDB International expects that the outcome of the health insurance negotiations will have a huge impact on the competition, pricing pattern and sales of innovative drugs in the next 1-2 years.
China's new drug research and development continues to develop. China's innovative drug approval has entered the fast lane since 2017, and the number of therapeutic INDs reached 555 in 2020, an increase of 252 compared with 2019.
The situation of domestic innovative drugs is more serious, and PD-1 is a typical representative. Although the number of domestic new drug pipelines has increased significantly, there are a large number of situations with the same target from the perspective of target distribution, resulting in a fierce competition pattern for new drugs to be listed, and new drugs are no longer new.
According to cde's clinical analysis report of new anti-tumor drugs, the homogenization of new drug targets is very obvious, especially tumor hotspots such as PD-1 (L-1).
The policy promotes the elimination of low-level repetitive innovation by promoting clinical value orientation. In November 2021, cde issued the "Guidelines for Clinical Research and Development of Clinically Value-Oriented Antineoplastic Drugs", which puts forward higher requirements in the selection of controlled pharmacy/protocols, restricts a large number of repetitive or even me-worse innovative drugs to the clinic, and safeguards the interests of patients.
The innovative drug industry is experiencing the second industry clearance, and innovative drugs with clinical value and barriers are the future. As research and development continues to advance, the crowding of the me-too track makes the product face fierce competition after the launch, and will face a reshuffle again.
In the second reshuffle stage, Ping An Securities believes that it should pay attention to independent innovation enterprises with sustainable development potential in the future, as well as innovative drug products with high clinical value and barriers.
Orient Securities: Innovation must be the core direction to promote the further development of the pharmaceutical industry, and it is also the eternal theme of the industry. However, the definition of innovation in the future will be higher and higher, and some pseudo-innovations that repeat targets and pile up research in the past will be gradually eliminated, and for products with real barriers, products with uniqueness are expected to enjoy a higher valuation premium.
Local innovation is accelerating, and internationalization is becoming a trend. As of November 2021, a total of 23 innovative drug varieties have been approved for marketing. From 2015 to 2021, the approval of new drugs of class 1 in China continued to reach a new high, the number of NDA of class 1 new drugs remained high, the progress of internationalization of innovative drugs accelerated, and the number of MRCT continued to grow, highlighting the trend of localization innovation and internationalization.
2022 will be a key year for China's innovative drugs to go to sea. In 2019, B&E's Zambitinib became the first DOMESTICally approved innovative drug approved by the FDA, after which companies such as Innovent Biologics, Junshi Biologics, Kangfang Biologics, Legendary Biologics, and Huang Pharmaceutical submitted their respective BLA/NDA for innovative drugs, and the FDA will make approval decisions on these applications in 2022.
Compared with the China Food and Drug Administration, the FDA's new drug evaluation system and standards are more mature, and the requirements for product clinical data are often higher, and only the products and targets that really stand out can be favored by them.
The ability to innovate and go to sea is continuously improving, and high-quality innovation has been verified by the international market. Chinese pharmaceutical companies are at a critical point in the evolution from license-in overseas innovative drug assets to license-out independent research and development results.
License-out projects are beginning to enter a blowout period: since 2020, there have been 11 overseas license-out projects of more than HALF A billion US dollars, compared with a maximum of 1-2 per year, further proving the value of high-level innovation;
Entering 2022, there are still many heavy assets with out-licensing potential, such as a variety of dual antibody assets of Kangfang Bio and Corning Jerry, other indications of Nuocheng Jianhua Olibutinib, and Connoa's self-free pipeline.
Ten years of grinding a sword, the inflection point of China's innovation internationalization has arrived!
SPDB International: Plate differentiation in 2022 may continue, but innovation is an eternal theme. Standing at the current point in time, stock selection should not only adhere to the optimism of really strong innovation, but also pay attention to the valuation and the cost performance of the business itself.
Wanlian Securities: In 2022, the pharmaceutical industry may still face problems such as the decline in performance related to the epidemic, the tightening of medical insurance policies, and the homogenization of innovation competition, but in the long run, the pharmaceutical innovation industry chain may be a rare layout opportunity.
Dongxing Securities: The direction of innovation remains unchanged. Innovative drugs are expected to usher in a round of big waves, and enterprises with continuous competitiveness will adjust the pace and achieve leapfrog development through innovation, upgrading and internationalization.
Hengrui Pharmaceutical (600276. SH): The business inflection point is coming quickly, accelerating internationalization
(1) Innovative drugs were approved for medical insurance, and the proportion of innovative drugs increased significantly (CDK4/6, AR, PDL1, etc. will be approved this year and next year);
(2) New technology platforms, new therapeutic fields, and new target products are authorized to the outside world, and high milestones (XDC, antiviral, etc.) are obtained. (3) Overseas progress of innovative drugs, Apatinib + PD1 global multi-center first-line liver cancer is expected to be BLA in 2022, and pirlotinib lung cancer is expected to be NDA in 2022. Fluzopali, IL-17 monoclonal antibody, JAK1 and other products overseas key clinical Phase 3.
(4) The business inflection point will soon arrive, and the quarterly performance of the most influential collection and collection has begun to be reflected. It is expected that by the beginning of 2022Q3, the company's operating situation will gradually improve, the collection and procurement of generic drugs will be basically reflected, and the performance of innovative drugs will accelerate.
Simcere Pharmaceuticals (2096. HK): Firm innovation, forge ahead
(1) The proportion of innovation has increased significantly. Anti-tumor, central nervous system, and autoimmunity are the main layout directions of the company's R&D pipeline, and the proportion of the company's innovative drug sales revenue will increase significantly (it is expected to account for about 60% in 2021);
(2) Stage of rapid growth of performance. With the development of new indications for Endau, the rapid release of FirstBixin and Edexin medical insurance, the company's performance in the next 3 years is a stage of rapid growth;
(3) R & D and BD R & D and BD dual drive, pipeline differentiation layout.
Junshi Bio (688180. SH): A rare biotech with source innovation capabilities
(1) Numerous First-in-class preclinical molecular reserves to continuously empower innovation and development;
(2) Outstanding clinical research and development capabilities, synchronous promotion and efficient output of multiple large-scale key clinics;
(3) Leading the progress of internationalization, JS016 took the lead in commercialization in the United States, bringing sustainable income;
(4) There is a large expectation difference in R&D capabilities and internationalization progress, and it is expected to continue to exceed expectations.
Beida Pharmaceuticals (300558. SZ) A share scarce innovative drug target, internationalization is imminent.
(1) Exetinib continues to contribute stable cash flow, and ensartinib's new medical insurance is expected to be rapidly increased, enriching cutting-edge pipelines to promote clinical practice;
(2) The R&D team has overseas R&D experience + excellent R&D strength, and the sales team has strong strength;
(3) Equity incentives set a sales target of 8 billion yuan in 2025, reflecting the company's strong confidence.
Kingsley Biotechnology (1548. HK): Multi-sector layout helps accelerate growth
(1) The business layout of Kingsway is more forward-looking, and the revenue side has accelerated significantly in recent years. The existing business covers four major areas: life science services and products, biopharmaceutical CDMO, industrial synthetic biology and cell therapy.
(2) The life science business is the basic disk for the company's development, and the scale of technology-driven related revenue continues to expand, providing stable cash flow.
(3) The vigorous biological CDMO track is of high quality, and the expansion of production capacity supports the expansion of income scale.
(4) The core products of the subsidiary's legendary biological cell therapy are about to be commercialized and rich in research pipelines.
Rongchang Biology (9995. HK): In 2021, two new products will be included in the medical insurance catalogue in the medical insurance negotiations
With the help of medical insurance coverage and large-scale hospital sales, the company's two blockbuster new drugs listed in 2021 will usher in considerable release in 2022.
(Source of the report: SPDB International, China Development Bank Securities, Huaxi Securities, Guosheng Securities, Orient Securities, Dongxing Securities, Galaxy Securities, Ping An Securities, Guosheng Securities, Zheshang Securities; the information in this article does not constitute any investment advice, the content published is from licensed securities institutions, does not represent the views of the platform, please judge and make decisions independently of investors.) )
For more information, please download the 21 Finance APP