Previous Episode Review:
Most of the organic chemistry is actually the neutralization reaction of Lewis acid and Lewis base, and electrons flow from electron-rich places to electron-deficient places.
The first contact with electrophilic addition is not unexpected when I was learning olefins
First of all, electrophilic and nucleophilic are relatively simultaneous, hydrogen protons to bind π electrons, that is the process of electrophilic, if in turn, π electrons to bind hydrogen protons, it is a nucleophilic process (although we never seem to say [doge])
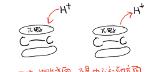
When electrophilic addition occurs between olefins and different reagents, it can be carried out according to the "carbocation intermediate mechanism", "ring positive ion intermediate mechanism", "ion-to-intermediate mechanism" and "three-center transition state mechanism".
Don't panic, let's see one by one.
In this issue, let's take a look at the "mechanism of carbon positive ion intermediates"
Represented is the reaction of olefins and hydrohalic acids.
To simplify it again, let's look at the ethylene and HCl reactions.
First of all, for an ethylene, the π electrons are evenly distributed on both sides.
When the hydrogen proton obtained by HCl heterocracking appears around the olefin, the π electron will move toward the hydrogen proton, after all, the positive and negative charges attract each other, the moth puts out the fire, at this time the π electron cloud will undergo a certain degree of deformation, and then when the π electron cloud and the s-space orbit of the hydrogen proton overlap, a C-Hσ bond will be formed.
Originally, the two electrons belonged to the two carbons in common, at this time only belonged to one of the carbons, and the other carbon had no electrons, leaving an empty p-orbit, positively charged. We call it "carbon positive ions."
Then, when there are chloride ions around this carbocation, the outermost electrons of chlorine are also deformed by the attraction of carbon positive ions, and then overlap with the empty p-orbital of the carbon positive ions to form the C-Clσ bond.
Since intermediates such as carbon positive ions are formed in this reaction, we call this mechanism "carbon positive ion intermediate mechanism". Isn't that hard?
Next, a little more complicated, what about propylene and HCl addition products?
Don't panic, when the ethylene link has a methyl group, due to the carbon sp3 hybridization of the methyl, the double bond carbon sp2 hybridization, the electronegativity sp2 is greater than sp3, so the methyl group gives electrons, and the induction effect of +I. This causes the double-bonded π electron cloud to move away from the methyl group, that is, in the absence of hydrogen protons π electrons have moved in advance.
To simplify, without drawing an electron cloud, we can mark the electrical properties carried by each carbon.
At this time, if there are hydrogen protons around, they will definitely form a C-Hσ bond with Carbon 1 with δ-. Chloride ions and carbon 2 with δ+ form the C-Clσ bond.
Seeing this, what do you think of?
Yes, the Martens rule, hydrogen is added to the hydrogen-rich carbon.
So, what if you replace -CH3 with -CF3?
The following scenario should be easily envisioned.
So it is the opposite of the addition of HCl, that is, the anti-Martens addition product is obtained.
As we mentioned earlier, different carbocation stability is a bit different.
Does this have anything to do with the electrophilic bonus?
Yes! The stakes are big.
For example, when we react with the following olefins and HCl, it is reasonable to say that we can get the product of double bond addition.
But the experiment hit the face, and we got another compound.
If you look closely, the front Cl is connected to a secondary carbon, while the one below is connected to a tertiary carbon.
How??
This brings us to the rearrangement of carbon positive ions
Because the σ bond orbit of C-CH3 can overlap with the p orbit of the carbon positive ion when it is turned to a certain angle, and then the methyl group runs with a pair of electrons to defect and bond with the carbon positive ion. Leaving a three-stage carbocation wait and chloride ions bonding.
Change it a little bit more.
What is the product of this reaction?
Give you 5 seconds to think.
5
4
3
2
1
The answer is C.
Hey hey, hydrogen with a pair of electrons defected ~
This issue will go here first~
To be continued~
Resources:
1. Xing Qiyi, Pei Weiwei, Xu Ruiqiu, Pei Jian. Basic Organic Chemistry (Fourth Edition), Higher Education Press.
Thank you for your attention, like, share ~