Source | Cyber blue
Edit | Koharu
Well-known pharmaceutical companies pass the consistency evaluation
Recently, the State Food and Drug Administration released the latest drug approval documents, a total of 14 varieties, 24 product specifications passed / deemed to pass the consistency evaluation. It includes omeprazole sodium for injection, atorvastatin calcium tablets, apixaban tablets, siglitine phosphate tablets, pregabalin capsules, irbesartan tablets and other large varieties.
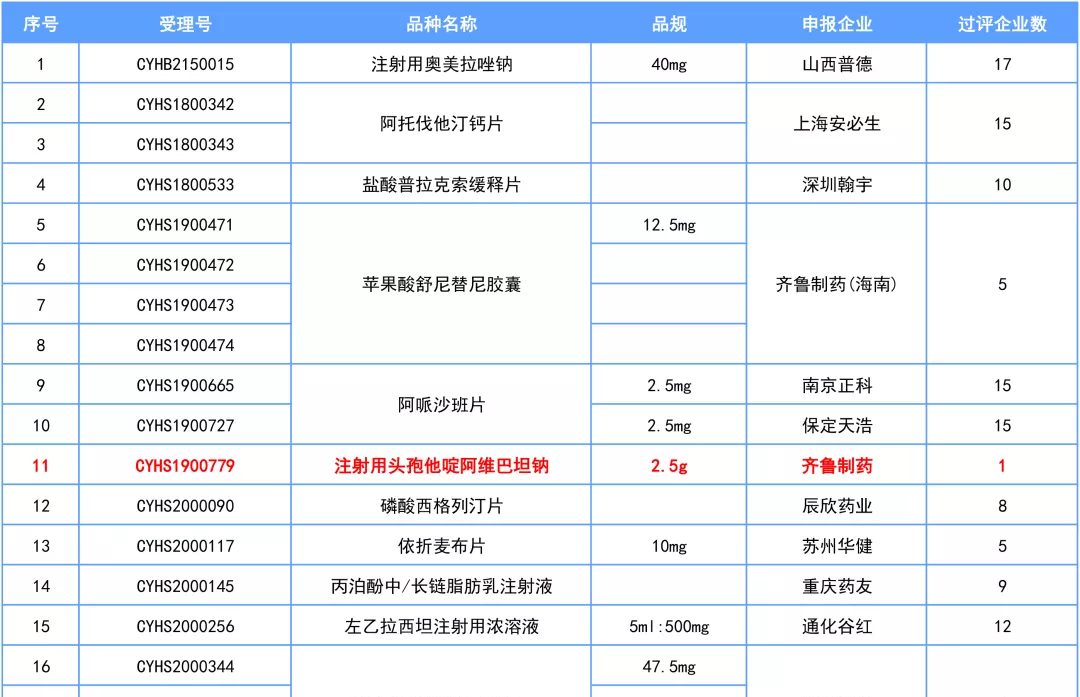
Image source: Medicine Spring and Autumn
01
Soared 981.89% of the varieties have been evaluated
Racoxamide tablets
The successful approval of the consistency evaluation of racoxamide tablets this time is Shisi Pharmaceutical Group.
Lacosamide is a novel N-methyl-D-aspartate (NMDA) receptor glycine site binding antagonist developed by Unisby, which is an anticonvulsant drug with a new dual mechanism of action, suitable for monotherapy and combination therapy of partial seizures in patients with epilepsy aged 4 years and older.
On October 11, Shisi Pharmaceutical Group issued an announcement that it had obtained the approval of the State Drug Administration for the production registration of racoxamide tablets (50mg and 100mg), which belonged to the new drugs of chemical drugs class 4 and were deemed to have passed the consistency evaluation.
According to Unisex's financial report, the global sales of racoxamide in 2020 were 1.656 billion US dollars, and the data of the intranet of The Minenet shows that the total sales of racoxamide in China's public medical institutions and Chinese urban physical pharmacies terminals in 2020 were 37.76 million yuan. Due to the small base, the sales of racoxamide terminals in urban physical pharmacies in China rose by 981.89% in 2020, an obvious increase.
The market space for racoxamide is vast.
It is reported that epilepsy has become the second most common disease in Chinese neurology after headache, with about 9 million epilepsy patients in China and about 600,000 new patients every year. Moreover, the treatment of epilepsy requires a long cycle, or even a lifetime of medication. Racoxamide tablets have certain uniqueness, small side effects, do not go through liver metabolism, and do not produce clinically significant drug interactions with other anti-epileptic drugs.
In November 2018, Unisby's racoxamide tablets were approved for listing in China, and in January 2019, Jiangxi Qingfeng Pharmaceutical won the first domestic imitation of racoxamide tablets.
In May this year, Buchang Pharmaceutical's laroxamide tablets became the second domestic one, and Beijing Sihuan Pharmaceutical and Shijiazhuang Siyao were also evaluated this year. In addition, Hefei Yifan Biological, Changzhou Pharmaceutical, Shandong Bainuo Pharmaceutical, Hefei Yingtai Pharmaceutical and other enterprises also have the layout of this product.
02
Several large varieties have been evaluated
Apixaban tablets
The people who successfully won the approval of the consistency evaluation of the apixaban tablets this time were Nanjing Zhengke and Baoding Tianhao.
Apixaban is a highly selective direct factor Xa inhibitor jointly developed by Squibb and Pfizer primarily in adult patients undergoing elective hip or knee replacement to prevent venous thromboembolic events. In 2020, the global sales of Squibb apixaban tablets exceeded $9.168 billion, ranking in the top 10 of global drug sales.
Apixaban was first approved for listing in China in 2013 and has seen rapid sales growth in recent years.
According to data from the intranet, in 2020, the sales of Apixaban, the terminal of China's public medical institutions, exceeded 100 million yuan, an increase of 104.8% year-on-year.
It is reported that apixaban tablets have been included in the third batch of collection, Qilu Pharmaceutical, Haosen Pharmaceutical, Jiayi Pharmaceutical and Chia Tai Tianqing Pharmaceutical and other 4 enterprises won the bid and divided the market. The third batch of national collection and procurement has entered the formal procurement stage from October and November 2020.
It is worth noting that according to the Pharmaceutical Economic News, after the implementation of the landing procurement, the sales volume of the product in the fourth quarter increased slightly compared with the third quarter, but the sales amount fell sharply, with a decline of 64.0%.
Image source: Medical Economics
At present, nearly 20 enterprises have been approved for production and regarded as the same as the evaluation, and there are more than 30 layout enterprises. Kelun Pharmaceutical, Qilu Pharmaceutical, Dongguang Pharmaceutical, Beite Pharmaceutical, Nanjing Chia Tai Tianqing and other enterprises have been evaluated.
As more and more enterprises have been evaluated, unsolicited enterprises and newly approved enterprises are gradually entering the provincial collection platform, snatching away about 30% of the remaining amount of other price-appropriate hanging net varieties purchased by various public medical institutions for provincial drug collection. It is foreseeable that the form of competition for related products will become more intense.
Sunitinib malate capsules
The successful approval of the consistency evaluation of sunitinib malate was Qilu Pharmaceutical.
Sunitinib is a multi-target receptor tyrosine kinase inhibitor developed by Pfizer, which is clinically mainly used for the treatment of adult patients with gastrointestinal stromal tumors, renal cell carcinoma and pancreatic neuroendocrine tumors.
According to Intranet data, Pfizer's shunitinib malate capsules peaked in global sales of $1.236 billion in 2012 and $819 million in 2020. In the domestic market, sunitinib as a national medical insurance class B variety in 2020, the terminal sales of public hospitals in key provinces and cities in China in 2020 will be 136 million yuan, an increase of 39.3% year-on-year.
At present, only the original Pfizer products are sold in the market, but with the expiration of the patent for the product, its market pattern will be re-divided.
It is reported that the patent of "Sunitinib" in China expired as early as 2021, and many domestic companies have also begun to actively layout this field, including CSPC Pharmaceutical Group (Shoufang), Howsen Pharmaceutical, Chia Tai Tianqing, Qilu Pharmaceutical, and Kelun Pharmaceutical.
Sitriglitine phosphate tablets
The person who successfully obtained the approval of the consistency evaluation of sitagliptin phosphate this time is Chenxin Pharmaceutical.
Sieglitine is the world's first DPP-4 inhibitor, compared with traditional hypoglycemic drugs, with no increased risk of hypoglycemia, neutral effect on weight, good cardiovascular safety and other advantages, according to Merck's financial report, merck's global sales of strigliptin phosphate in 2020 is 3.306 billion US dollars.
The scale of the product in the domestic market also showed a clear upward trend.
In 2009, Merck's siglitine phosphate tablets entered The Country, entered the National Medical Insurance (Class B) in 2017, and successfully renewed the contract in 2020, taking advantage of the advantages of medical insurance to quickly release the volume. According to the data of the medical cube, the sales amount of city public hospitals and county-level public hospitals such as sitaglitine phosphate tablets (trade name: Genevi) in 2020 is 1.172 billion yuan.
According to the medicine Spring and Autumn combing, in addition to 8 domestic enterprises such as Merck Shadong, Chia Tai Tianqing, CSPC Ouyi, Yangtze River Pharmaceutical and Chenxin Pharmaceutical, there are 9 manufacturers such as Qilu, Kelun, Sandoz, and Dr. Ruidi of India to report production in the new 4/5.2 category.
In addition to sigueliptin, there are currently 4 types of DPP-4 inhibitors that have been approved for marketing in China, namely saxagliptin, viloggliptin, lifiglitine and alogliptin.
According to the data of the intranet, in 2019, the sales performance of the five DPP-4 inhibitors of the terminal of China's public medical institutions were good, and the sales of lifiglitine tablets led the run with a sales growth of 104.17%, and the sales of alogretine benzoate tablets are also about to cross the 100 million yuan mark.
According to the statistics of Industrial Securities, in 2019, the market size of diabetes drugs in China was about 53.98 billion yuan, and the market size of DPP-4 inhibitors was about 4.965 billion yuan, accounting for 9.2% of the overall market share of hypoglycemic drugs. The global market size of DPP-4 inhibitors is about 8.67 billion US dollars, accounting for 17% of the overall antidiabetic drug market.
Compared with the international market, it can be seen that the proportion of DPP-4 inhibitors in China in hypoglycemic drugs still has room to increase, and the market scale is expected to be further expanded.
03
Consistency evaluation is growing rapidly
At present, the development of the consistency evaluation policy has been relatively mature, and the number of evaluated products has increased rapidly.
According to Flint Creation statistics, as of October 8, 2021, the OFFICIAL WEBSITE OF THE CDE disclosed that a total of 3320 drug consistency evaluation acceptance numbers were undertaken in September, and a total of 51 acceptance numbers were newly undertaken. As of October 8, 2021, a total of 2964 drugs listed on the product specification passed the consistency evaluation (including 1182 product specifications), and a total of 69 products passed the consistency evaluation drug (including 27 product specifications) this month, and after weight removal by dosage form, a total of 40 varieties were introduced.
Image source: Flint Creation
Such development results are inseparable from the policy support of the state.
In 2012, the Concept of Consistency Evaluation was first proposed in the 12th Five-Year Plan for National Drug Safety.
In 2018, the State Drug Administration issued the "Announcement on Matters Related to the Consistency Evaluation of the Quality and Efficacy of Generic Drugs", which pointed out that the 2018 version of the National Basic Drug Catalogue established a dynamic adjustment mechanism, and the varieties that passed the consistency evaluation of the quality and efficacy of generic drugs were given priority to be included in the catalog, and the varieties that did not pass the consistency evaluation would be gradually transferred out of the catalog. Link consistency evaluation policies to basic drug lists.
In 2019, the General Office of the State Council issued the "Pilot Program for centralized procurement and use of drugs organized by the State", and 22 of the 25 shortlisted varieties passed the consistency evaluation, and the consistency evaluation policy was once again closely linked to the largest IP in the medical reform, "with quantity procurement".
In this context, domestic enterprises have promoted consistency evaluation.
Among the products that have passed the consistency evaluation this time, there are products that have been included in the purchase of quantity, and the consistency evaluation of the evaluation is the premise for the enterprise to maintain the hanging network, and there are also potential varieties that have not yet expired the patent period, and the enterprise can seize the market at the first time after the expiration of the original patent period through the consistency evaluation.
All in all, the consistency evaluation of drugs has become a key point that enterprise development has to pay attention to.