The construction of animal laboratories is also called the construction of laboratory animal houses, because it is for the construction of buildings suitable for breeding and breeding laboratory animals. Such building construction should have specific environmental requirements and experimental means to ensure the quality of animals and the accuracy and reliability of experimental research. According to the degree of control of microorganisms, it can be divided into: open system, barrier system and isolation system.
First, SPF level animal experimental room
Meet the requirements of animal habitation, strictly control the entry and exit of people, goods and air, and are suitable for breeding laboratory animals of the clean or specific pathogen free (SPF) level. After being tested by a professional monitoring center and passing the barrier environment accepted by experts on site, it can be used as a feeding environment for clean grade or SPF grade animals.
Second, laboratory animal room| animal laboratory | animal research room classification and standards
According to the laboratory animal microbial control standards, the laboratory can divide the laboratory animals into four levels, namely ordinary animals, cleaning animals, animals without special pathogens, sterile or habitat animals.
1. Class I Common Animal (CV)
Corresponding grade requirements: refers to general animals whose microorganisms are not under special control. It is required to exclude pathogens from zoonotic diseases and pathogens from a very small number of virulent infectious diseases in laboratory animals. In order to prevent infectious diseases, when breeding and breeding experimental animals, certain measures should be taken to ensure that the results they use for testing are reproducible (that is, experiments done by animals of the same strain at different times with the same strain of animals in accordance with the prescribed experimental procedures can obtain almost the same results).
2. Secondary Cleaning Animal (CL)
Corresponding grade requirements: Require the exclusion of zoonotic diseases and pathogens of major infectious diseases in animals
3. Tertiary Animals Without Special Pathogens (SPF)
Corresponding grade requirements: In addition to the requirements to the second level, some prescribed pathogens should be excluded. Its sterilization and sterilization methods can be used for high-efficiency air filter sterilization method, ultraviolet sterilization method, triethylene glycol vapor spray method and lithium chloride aqueous solution spray method
4. Class IV Sterile Animals (GF) or Habitats (GN)
Corresponding grade requirements: Sterile animals are required not to carry any microorganisms that can be detected with existing methods. Habitats require the implantation of one or more known microorganisms on sterile animal bodies.
Pathological examination standards for three and four types of experimental animals
In terms of pathological examination, the four types of laboratory animals also have different pathological examination standards.
The first degree is healthy in appearance, and there should be no lesions in the main organs.
Secondary in addition to primary indicators, microscopic examination of lesions without secondary microbial pathogens.
Tertiary animals without special pathogens. There are no lesions of secondary and tertiary microbial pathogens.
Grade IV does not contain lesions of secondary and tertiary microbial pathogens, and the spleen and lymph nodes are sterile animal histological structures.
Fourth, the requirements of the environmental design of the animal house
For different levels of experimental animals, there are different requirements for the design and management of animal houses.
Sterile, known bacteria and animals without special pathogens need to be raised in a sterile or as sterile environment as possible, this environment, which is currently commonly known as the barrier environment in the world, that is, a barrier is used to separate the animal from the surrounding polluted environment, just like the fetal rat in the womb of the mother mouse.
This environment is divided into five categories from the perspective of controlling microorganisms, such as isolation systems, barrier systems, semi-barrier systems, open systems and laminar flow frame systems.
1. Isolate the system
Is a system for raising animals in containers with operating gloves for raising sterile animals and habitats. The interior maintains a level of 100 cleanliness according to microbiological requirements, but the room and operators in which it is set up do not have to be considered as a sterile room.
2. Barrier system
The sterile clean room of about 10,000 to 100,000 grades is used as a feeding room, which is mainly used for long-term breeding and breeding of animals without special pathogens. Strict management is implemented when entering the house, such as showering, changing intimate clothes, etc.
3. Semi-barrier system
Relax the management of people and things entering and leaving the room in the barrier system, and the plane composition is roughly the same as that of the barrier system.
4. Laminar flow rack system
The cage is placed in clean horizontal laminar flow air. It is often used for small-scale rearing, but there is a risk of contamination when raised, handled and handled in general rooms. Can be used for semi-barrier supplementation.
5. Open system
It is a system that does not eliminate pollution when people, things, air, etc. enter and leave the room, but usually requires some degree of cleaning management.
5. Site selection and architectural design of laboratory animal rooms
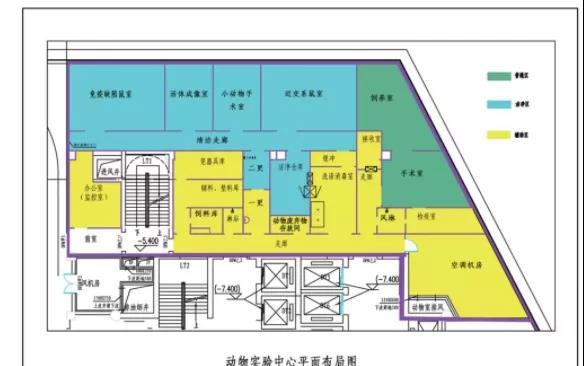
1. The construction of animal laboratories should be in a place where the environment is clean and quiet, the terrain is high and dry, the drainage and ventilation are good, and the supply of water and electricity is guaranteed.
2. Stay as far away from factories, bustling residential areas, slaughterhouses, livestock and poultry farms, and areas with the threat of epidemic sources and pollution.
Animal houses for production and scientific research units should be built separately in a small area, isolated from other departments.
3. The size of the cleaning preparation room must be determined according to the content of the work and the space occupied by facilities such as disinfection and sterilization devices.
4. The cleaning preparation room and the cleaning corridor are separated from the washing room by the wall, and the wall is never allowed to crack.
5. The ground should be flat and not slippery, non-seepage, non-leakage, resistant to chemical corrosion and wear resistance. Rooms with strict environmental requirements should also consider choosing materials with low dust emissions. The corners of the wall and ground should be sleek and angular.
6. The inner wall should be smooth, water-resistant, wear-resistant, and resistant to disinfectant corrosion. The angle between walls and walls, walls and ceilings or beams should be smooth and angular.
7. The roof is generally not subject to pressure, using thin cement board coated with waterproof materials, which can withstand water flushing and disinfection water corrosion.
8. The breeding room usually does not have an outer window, and there is no inner window between the feeding rooms to avoid interference.
9. The door of the feeding room should be sealed with aluminum alloy, and the opening direction should pay attention to the pressure difference between indoor and outdoor. The door between the barrier and the non-barrier should be set up with a device that cannot be opened unless certain conditions are met.
10. Wooden structures are generally only used as simple temporary buildings; mixed structures are mostly single-storey buildings. The integrity of the reinforced concrete frame structure is good, the deformation is small, and it should be used in multi-storey buildings. The enclosure structure of steel skeleton, galvanized corrugated metal plate, and inlaid polystyrene rigid foam plastic sheet as insulation layer has good airtight insulation performance.
Guangzhou Jiadong, a professional laboratory service provider, has laboratory construction qualifications, laboratory related patents; our core technology is mainly laboratory planning and design, production and installation, construction and decoration and accreditation and other all-round one-stop services, for you to create a scientific, comfortable, safe laboratory is our pursuit.
Disclaimer: Articles and pictures are from the Internet, such as invasion and deletion!