Original Katsura branch rice inner mesh
Great content
Recently, Lepu Pharmaceutical has entered the administrative approval stage with generic 4 types of valsartan amlodipine tablets (I.) and is expected to be approved for listing in the near future. Valsartan amlodipine tablets (I.) are compound antihypertensive drugs, with total sales of more than 2.5 billion yuan in 2020 in china's public medical institutions and china's urban physical pharmacies.
Valsartan amlodipine tablets (I.), originally developed by Novartis, are a monolithic compound formulation composed of the angiotensin receptor antagonist valsartan and the calcium channel antagonist amlodipine, which is suitable for patients with valsartan monotherapy or amlodipine monotherapy that fails to adequately control blood pressure.
According to data from the intranet, in 2020, the total sales of valartan amlodipine tablets (I.) in China's urban public hospitals, county-level public hospitals, urban community centers and township health centers (referred to as China's public medical institutions) and China's urban physical pharmacy terminals exceeded 2.5 billion yuan.
Whether in medical institutions or physical pharmacies, the market size of valsartan amlodipine tablets (I.) is increasing year by year. It is worth mentioning that in recent years, valsartan amlodipine tablets (I.) in China's urban physical pharmacy terminal sales growth rate of more than 20%, 2020 sales of 428 million yuan, an increase of 27.56% year-on-year, is expected to exceed 500 million yuan in 2021 sales.
Sales of Valsartan amlodipine tablets (I.) at the terminal of physical pharmacies in China (unit: 10,000 yuan)
Source: Intranet China's urban physical pharmacy terminal competition pattern
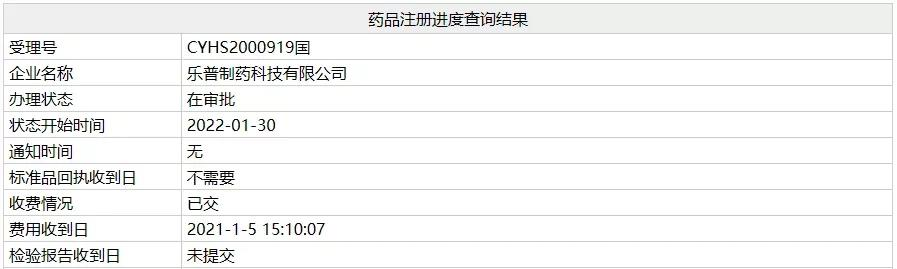
Although there are 9 local enterprises in China that have mass-produced valartamlodipine tablets (I.) and regarded as overrated, there are still more than 10 pharmaceutical companies that have applied for the listing of the product under review, and the registration status of the product of Lepu Pharmaceutical has been changed to "under approval", and if it is successfully approved, it will be regarded as over-evaluated.
Source: Intranet Database, NMPA
Note: The Minnei Network China Urban Physical Pharmacy Terminal Competition Pattern Database is an enlarged version of the urban physical pharmacy database covering 297 urban physical pharmacies in prefectures and cities and above (excluding county and rural physical pharmacies) across the country and continuously monitoring all categories. The above sales are calculated based on the average retail price of the product at the terminal.