The "most expensive drug in history" will land
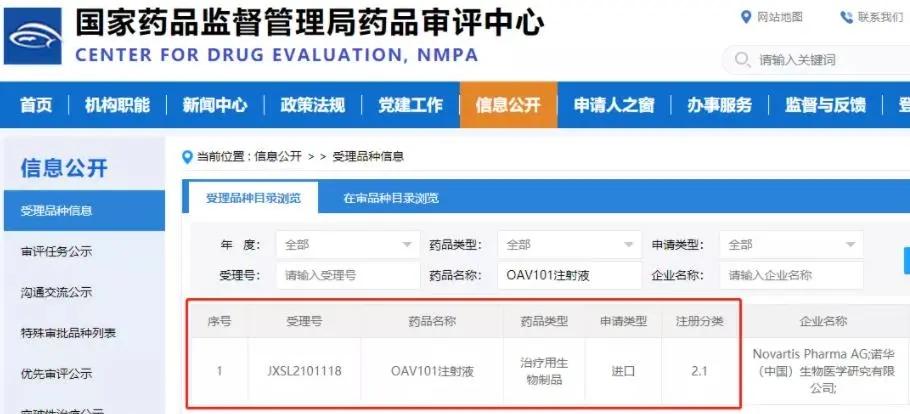
Recently, the Drug Evaluation Center (CDE) of the State Food and Drug Administration of China announced that the clinical trial application submitted by Novartis' AAV gene therapy drug Zolgensma (OAV101 injection) for the treatment of spinal muscular atrophy (SMA) has been tacitly licensed for clinical trials in China. Previously, on October 21, 2021, the clinical trial application submitted by the drug was accepted.
Only one intravenous dose is required
Achieve long-term relief or even cure
Speaking of ice bucket challenges, many people can think of the phrase "frozen people (amyotrophic lateral sclerosis, also known as ALS)."
But when it comes to its "brother disease" - SMA, many people are confused. In fact, SMA is also a motor neuron disease. After suffering from SMA, the motor nerve cells that control muscle activity do not work properly, and patients gradually lose various motor functions and even cannot breathe and swallow.
In newborns, the incidence of SMA is 1 in 6,000 to 1 in 10,000, and about 1 in 40 to 50 people in the conventional population is a carrier of the SMA pathogenic gene.
In May 2018, SMA was included in the First Catalogue of Rare Diseases jointly formulated by the National Health Commission and other departments.
Based on the patient's onset time and the maximum motor function that can be achieved, SMA is mainly divided into four types:
Type I is the most common and severe, with children often facing greater physical challenges and significant differences in their life cycles based on a number of factors;
Patients with types II, III, and VI tend to have a normal or near-normal life cycle, but their physical functioning changes over time.
As cruel as "frostbite people", SMA patients will continue to lose the corresponding motor function, but "gradual frost people" are generally adults, and SMA is generally infancy. And SMA is the earlier the onset of the disease, the more serious the disease, "gradually freezing people" is just the opposite.
On May 24, 2019, the U.S. FDA approved Novartis' first gene therapy for the treatment of SMA patients under 2 years of age, Zolgensma. Patients only need to receive one intravenous administration to express smN proteins in cells for a long time, achieving long-term remission and even cure.
Sky-high prices have caused great controversy
Currently priced at $2.125 million, Zolgensma is touted as the most expensive drug in pharmaceutical history. At present, Zolgensma has been approved in nearly 40 countries and regions around the world.
With a price of up to $2.125 million, some have questioned Novartis' threat to the lives of its children.
In response to social and public skepticism, Novartis CEO once said: "Those critics do not really think about how our medical system works. We transplant patients, and we spend $3 million to $5 million per patient, but they don't work as well as these drugs. The CEO said that if the therapy is performed only once, the cost is cheaper than the current therapy.
Cases of sky-high drug "floor price" insurance
Not easily copied.
At present, 3 SMA therapies have been approved worldwide, in addition to Zolgensma, but also Bojian Spinraza and Roche Evrysdi, the latter two have been approved in China.
Spinraza (Northinacine sodium injection) was approved by the State Food and Drug Administration in February 2019 for the treatment of 5q spinal muscular atrophy (accounting for about 95% of all SMA cases), which is also the first drug approved to treat SMA in China.
Previously, Spinraza was priced at nearly 700,000 bottles in China, requiring repeated injections every year and being a completely self-funded drug.
In the 2021 National Medicare Drug List negotiations, 7 drugs for rare diseases were included in the Medical Insurance List, including spinraza, which is highly concerned for the treatment of spinal muscular atrophy.
2021 National Medical Insurance Catalogue Drug Negotiation Site
January 1, 2022, is the day when the 2021 version of the National Medical Insurance Drug List will be implemented. The price of Spinraza single needle has dropped from 700,000 yuan to more than 30,000 yuan. According to data from the Mei'er SMA Care Center, on January 1 this year alone, more than 20 SMA patients in 11 provinces have used Spinraza.
Although Spinraza's sharp price reduction has given hope to many rare disease patients, an industry insider admitted that Spinraza's price reduction is "(price) unexpected, (insured) reasonable".
"Spinraza is not a new drug, it has been basically profitable in the global market, the number of SMA children in China is large, and the drug market is very large. Bojian is actually answering the question of whether to seize the Chinese market in the face of the price reduction of medical insurance negotiations. ”
According to the analysis of the above-mentioned industry insiders, the entry of rare disease drugs into the medical insurance list is essentially under the established medical insurance negotiation framework, due to the breakthrough of individual cases reached by enterprise price reductions, and Bojian has voted for China in the strategic choice.
Disclaimer: This article is reproduced content, the copyright belongs to the original author, the purpose of reprinting is to convey more information, and does not represent the views of this platform. If it involves the content of the work, copyright and other issues, please contact the message of this official account, we will delete the content in the first time.